Impaired formation of high-order gephyrin oligomers underlies gephyrin dysfunction-associated pathologies
- PMID: 33532714
- PMCID: PMC7822942
- DOI: 10.1016/j.isci.2021.102037
Impaired formation of high-order gephyrin oligomers underlies gephyrin dysfunction-associated pathologies
Abstract
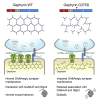
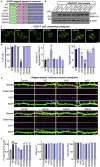
Gephyrin is critical for the structure, function, and plasticity of inhibitory synapses. Gephyrin mutations have been linked to various neurological disorders; however, systematic analyses of the functional consequences of these mutations are lacking. Here, we performed molecular dynamics simulations of gephyrin to predict how six reported point mutations might change the structural stability and/or function of gephyrin. Additional in silico analyses revealed that the A91T and G375D mutations reduce the binding free energy of gephyrin oligomer formation. Gephyrin A91T and G375D displayed altered clustering patterns in COS-7 cells and nullified the inhibitory synapse-promoting effect of gephyrin in cultured neurons. However, only the G375D mutation reduced gephyrin interaction with GABAA receptors and neuroligin-2 in mouse brain; it also failed to normalize deficits in GABAergic synapse maintenance and neuronal hyperactivity observed in hippocampal dentate gyrus-specific gephyrin-deficient mice. Our results provide insights into biochemical, cell-biological, and network-activity effects of the pathogenic G375D mutation.
Keywords: Molecular Biology; Neuroscience; Structural Biology.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Simultaneous impairment of neuronal and metabolic function of mutated gephyrin in a patient with epileptic encephalopathy.EMBO Mol Med. 2015 Dec;7(12):1580-94. doi: 10.15252/emmm.201505323. EMBO Mol Med. 2015. PMID: 26613940 Free PMC article.
-
Nanoscale Molecular Reorganization of the Inhibitory Postsynaptic Density Is a Determinant of GABAergic Synaptic Potentiation.J Neurosci. 2017 Feb 15;37(7):1747-1756. doi: 10.1523/JNEUROSCI.0514-16.2016. Epub 2017 Jan 10. J Neurosci. 2017. PMID: 28073939 Free PMC article.
-
Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons.J Neurosci. 2004 Jan 7;24(1):207-17. doi: 10.1523/JNEUROSCI.1661-03.2004. J Neurosci. 2004. PMID: 14715953 Free PMC article.
-
Gephyrin: a key regulatory protein of inhibitory synapses and beyond.Histochem Cell Biol. 2018 Nov;150(5):489-508. doi: 10.1007/s00418-018-1725-2. Epub 2018 Sep 27. Histochem Cell Biol. 2018. PMID: 30264265 Review.
-
Gephyrin: a central GABAergic synapse organizer.Exp Mol Med. 2015 Apr 17;47:e158. doi: 10.1038/emm.2015.5. Exp Mol Med. 2015. PMID: 25882190 Review.
Cited by
-
Gephyrin-Lacking PV Synapses on Neocortical Pyramidal Neurons.Int J Mol Sci. 2021 Sep 17;22(18):10032. doi: 10.3390/ijms221810032. Int J Mol Sci. 2021. PMID: 34576197 Free PMC article.
-
Synaptic Physiology Depends on Electrical Forces and Liquid-Liquid Phase Separation.Rev Physiol Biochem Pharmacol. 2025;187:339-359. doi: 10.1007/978-3-031-68827-0_17. Rev Physiol Biochem Pharmacol. 2025. PMID: 39838018 Review.
-
Is the Activation of the Postsynaptic Ligand Gated Glycine- or GABAA Receptors Essential for the Receptor Clustering at Inhibitory Synapses?Biomedicines. 2025 Aug 5;13(8):1905. doi: 10.3390/biomedicines13081905. Biomedicines. 2025. PMID: 40868159 Free PMC article. Review.
-
Phosphoinositide- and Collybistin-Dependent Synaptic Clustering of Gephyrin.J Neurochem. 2025 Aug;169(8):e70169. doi: 10.1111/jnc.70169. J Neurochem. 2025. PMID: 40781785 Free PMC article.
-
Neurodevelopmental Disorders in Patients With Complex Phenotypes and Potential Complex Genetic Basis Involving Non-Coding Genes, and Double CNVs.Front Genet. 2021 Sep 21;12:732002. doi: 10.3389/fgene.2021.732002. eCollection 2021. Front Genet. 2021. PMID: 34621295 Free PMC article.
References
-
- Agarwal S., Tannenberg R.K., Dodd P.R. Reduced expression of the inhibitory synapse scaffolding protein gephyrin in Alzheimer's disease. J. Alzheimers Dis. 2008;14:313–321. - PubMed
-
- Alvarez F.J. Gephyrin and the regulation of synaptic strength and dynamics at glycinergic inhibitory synapses. Brain Res. Bull. 2017;129:50–65. - PubMed
-
- Bath K.G., Lee F.S. Variant BDNF (Val66Met) impact on brain structure and function. Cogn. Affect. Behav. Neurosci. 2006;6:79–85. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

