This is a preprint.
Antibody Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7
- PMID: 33532778
- PMCID: PMC7852271
- DOI: 10.1101/2021.01.25.428137
Antibody Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7
Update in
-
Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7.Nature. 2021 May;593(7857):130-135. doi: 10.1038/s41586-021-03398-2. Epub 2021 Mar 8. Nature. 2021. PMID: 33684923
Abstract
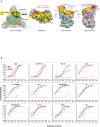
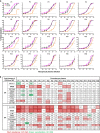
The COVID-19 pandemic has ravaged the globe, and its causative agent, SARS-CoV-2, continues to rage. Prospects of ending this pandemic rest on the development of effective interventions. Single and combination monoclonal antibody (mAb) therapeutics have received emergency use authorization1-3, with more in the pipeline4-7. Furthermore, multiple vaccine constructs have shown promise8, including two with ~95% protective efficacy against COVID-199,10. However, these interventions were directed toward the initial SARS-CoV-2 that emerged in 2019. The recent emergence of new SARS-CoV-2 variants B.1.1.7 in the UK11 and B.1.351 in South Africa12 is of concern because of their purported ease of transmission and extensive mutations in the spike protein. We now report that B.1.1.7 is refractory to neutralization by most mAbs to the N-terminal domain (NTD) of spike and relatively resistant to a few mAbs to the receptor-binding domain (RBD). It is not more resistant to convalescent plasma or vaccinee sera. Findings on B.1.351 are more worrisome in that this variant is not only refractory to neutralization by most NTD mAbs but also by multiple individual mAbs to the receptor-binding motif on RBD, largely due to an E484K mutation. Moreover, B.1.351 is markedly more resistant to neutralization by convalescent plasma (9.4 fold) and vaccinee sera (10.3-12.4 fold). B.1.351 and emergent variants13,14 with similar spike mutations present new challenges for mAb therapy and threaten the protective efficacy of current vaccines.
Figures






References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
