This is a preprint.
SARS-CoV-2 infection in pregnancy is associated with robust inflammatory response at the maternal-fetal interface
- PMID: 33532791
- PMCID: PMC7852242
- DOI: 10.1101/2021.01.25.21250452
SARS-CoV-2 infection in pregnancy is associated with robust inflammatory response at the maternal-fetal interface
Update in
-
Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface.Med. 2021 May 14;2(5):591-610.e10. doi: 10.1016/j.medj.2021.04.016. Epub 2021 Apr 30. Med. 2021. PMID: 33969332 Free PMC article.
Abstract
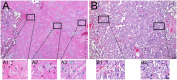
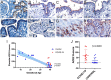
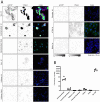
Pregnant women appear to be at increased risk for severe outcomes associated with COVID-19, but the pathophysiology underlying this increased morbidity and its potential impact on the developing fetus is not well understood. In this study of pregnant women with and without COVID-19, we assessed viral and immune dynamics at the placenta during maternal SARS-CoV-2 infection. Amongst uninfected women, ACE2 was detected by immunohistochemistry in syncytiotrophoblast cells of the normal placenta during early pregnancy but was rarely seen in healthy placentas at full term. Term placentas from women infected with SARS-CoV-2, however, displayed a significant increase in ACE2 levels. Using immortalized cell lines and primary isolated placental cells, we determined the vulnerability of various placental cell types to direct infection by SARS-CoV-2 in vitro. Yet, despite the susceptibility of placental cells to SARS-CoV-2 infection, viral RNA was detected in the placentas of only a subset (~13%) of women in this cohort. Through single cell transcriptomic analyses, we found that the maternal-fetal interface of SARS-CoV-2-infected women exhibited markers associated with pregnancy complications, such as preeclampsia, and robust immune responses, including increased activation of placental NK and T cells and increased expression of interferon-related genes. Overall, this study suggests that SARS-CoV-2 is associated with immune activation at the maternal-fetal interface even in the absence of detectable local viral invasion. While this likely represents a protective mechanism shielding the placenta from infection, inflammatory changes in the placenta may also contribute to poor pregnancy outcomes and thus warrant further investigation.
Figures


References
-
- Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641–7. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
