The Limit of Detection Matters: The Case for Benchmarking Severe Acute Respiratory Syndrome Coronavirus 2 Testing
- PMID: 33532847
- PMCID: PMC7929140
- DOI: 10.1093/cid/ciaa1382
The Limit of Detection Matters: The Case for Benchmarking Severe Acute Respiratory Syndrome Coronavirus 2 Testing
Abstract
Background: Resolving the coronavirus disease 2019 (COVID-19) pandemic requires diagnostic testing to determine which individuals are infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The current gold standard is to perform reverse-transcription polymerase chain reaction (PCR) on nasopharyngeal samples. Best-in-class assays demonstrate a limit of detection (LoD) of approximately 100 copies of viral RNA per milliliter of transport media. However, LoDs of currently approved assays vary over 10,000-fold. Assays with higher LoDs will miss infected patients. However, the relative clinical sensitivity of these assays remains unknown.
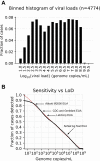
Methods: Here we model the clinical sensitivities of assays based on their LoD. Cycle threshold (Ct) values were obtained from 4700 first-time positive patients using the Abbott RealTime SARS-CoV-2 Emergency Use Authorization test. We derived viral loads from Ct based on PCR principles and empiric analysis. A sliding scale relationship for predicting clinical sensitivity was developed from analysis of viral load distribution relative to assay LoD.
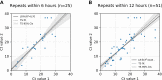
Results: Ct values were reliably repeatable over short time testing windows, providing support for use as a tool to estimate viral load. Viral load was found to be relatively evenly distributed across log10 bins of incremental viral load. Based on these data, each 10-fold increase in LoD is expected to lower assay sensitivity by approximately 13%.
Conclusions: The assay LoD meaningfully impacts clinical performance of SARS-CoV-2 tests. The highest LoDs on the market will miss a majority of infected patients. Assays should therefore be benchmarked against a universal standard to allow cross-comparison of SARS-CoV-2 detection methods.
Keywords: SARS-CoV-2; antigen detection; cycle threshold; limit of detection; viral load.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
-
- Smith KP, Cheng A, Chopelas A, et al. Large-scale, in-house production of viral transport media to support SARS-CoV-2 PCR testing in a multi-hospital healthcare network during the COVID-19 pandemic [manuscript published online ahead of print 23 July 2020]. J Clin Microbiol 2020. doi:10.1128/JCM.00913-20. - PMC - PubMed
-
- Abbott Molecular Inc. Abbott RealTime SARS-CoV-2 emergency use authorization package insert, REF 09N77-095, 51-608445/R1. Des Plaines, IL: Abbott Molecular Inc, 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

