The effect of tocilizumab on mortality in hospitalized patients with COVID-19: a meta-analysis of randomized controlled trials
- PMID: 33532896
- PMCID: PMC7853160
- DOI: 10.1007/s00228-021-03087-z
The effect of tocilizumab on mortality in hospitalized patients with COVID-19: a meta-analysis of randomized controlled trials
Abstract
Objective: We aimed to perform a meta-analysis of randomized controlled trials (RCTs) to summarize the overall effect of tocilizumab on the risk of mortality among patients with coronavirus disease 2019 (COVID-19).
Methods: We systematically searched PubMed, Cochrane Central Register of Controlled Trials, Google Scholar, and medRxiv (preprint repository) databases (up to 7 January 2021). Pooled effect sizes with 95% confidence interval (CI) were generated using random-effects and inverse variance heterogeneity models. The risk of bias of the included RCTs was appraised using version 2 of the Cochrane risk-of-bias tool for randomized trials.
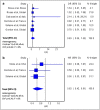
Results: Six RCTs were included: two trials with an overall low risk of bias and four trials had some concerns regarding the overall risk of bias. Our meta-analysis did not find significant mortality benefits with the use of tocilizumab among patients with COVID-19 relative to non-use of tocilizumab (pooled hazard ratio = 0.83; 95% CI 0.66-1.05, n = 2,057). Interestingly, the estimated effect of tocilizumab on the composite endpoint of requirement for mechanical ventilation and/or all-cause mortality indicated clinical benefits, with some evidence against our model hypothesis of no significant effect at the current sample size (pooled hazard ratio = 0.62; 95% CI 0.42-0.91, n = 749).
Conclusion: Despite no clear mortality benefits in hospitalized patients with COVID-19, tocilizumab appears to reduce the likelihood of progression to mechanical ventilation.
Keywords: Bias; COVID-19; Clinical trial; Mortality; Tocilizumab.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH, DE part of Springer Nature.
Conflict of interest statement
All named authors declare that they have no potential conflict of interest.
Figures
References
-
- Singh TU, Parida S, Lingaraju MC, Kesavan M, Kumar D, Singh RK (2020) Drug repurposing approach to fight COVID-19. Pharmacol Rep 72:1479–1508. https://doi.org/10.1007/s43440-020-00155-6 - PMC - PubMed
-
- Zhao M, Lu J, Tang Y, Dai Y, Zhou J Wu Y (2020) Tocilizumab for treating COVID-19: a systemic review and meta-analysis of retrospective studies. Eur J Clin Pharmacol. https://doi.org/10.1007/s00228-020-03017-5 - PMC - PubMed
-
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;l4898:366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

