BIO FOr CARE: biomarkers of hypertrophic cardiomyopathy development and progression in carriers of Dutch founder truncating MYBPC3 variants-design and status
- PMID: 33532905
- PMCID: PMC8160056
- DOI: 10.1007/s12471-021-01539-w
BIO FOr CARE: biomarkers of hypertrophic cardiomyopathy development and progression in carriers of Dutch founder truncating MYBPC3 variants-design and status
Abstract
Background: Hypertrophic cardiomyopathy (HCM) is the most prevalent monogenic heart disease, commonly caused by truncating variants in the MYBPC3 gene. HCM is an important cause of sudden cardiac death; however, overall prognosis is good and penetrance in genotype-positive individuals is incomplete. The underlying mechanisms are poorly understood and risk stratification remains limited.
Aim: To create a nationwide cohort of carriers of truncating MYBPC3 variants for identification of predictive biomarkers for HCM development and progression.
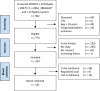
Methods: In the multicentre, observational BIO FOr CARe (Identification of BIOmarkers of hypertrophic cardiomyopathy development and progression in Dutch MYBPC3 FOunder variant CARriers) cohort, carriers of the c.2373dupG, c.2827C > T, c.2864_2865delCT and c.3776delA MYBPC3 variants are included and prospectively undergo longitudinal blood collection. Clinical data are collected from first presentation onwards. The primary outcome constitutes a composite endpoint of HCM progression (maximum wall thickness ≥ 20 mm, septal reduction therapy, heart failure occurrence, sustained ventricular arrhythmia and sudden cardiac death).
Results: So far, 250 subjects (median age 54.9 years (interquartile range 43.3, 66.6), 54.8% male) have been included. HCM was diagnosed in 169 subjects and dilated cardiomyopathy in 4. The primary outcome was met in 115 subjects. Blood samples were collected from 131 subjects.
Conclusion: BIO FOr CARe is a genetically homogeneous, phenotypically heterogeneous cohort incorporating a clinical data registry and longitudinal blood collection. This provides a unique opportunity to study biomarkers for HCM development and prognosis. The established infrastructure can be extended to study other genetic variants. Other centres are invited to join our consortium.
Keywords: Biomarkers; Hypertrophic cardiomyopathy; MYBPC3; Prognosis.
Conflict of interest statement
M. Jansen, I. Christiaans, S.N. van der Crabben, M. Michels, R. Huurman, Y.M. Hoedemaekers, D. Dooijes, J.D.H. Jongbloed, L.G. Boven, R.H. Lekanne Deprez, A.A.M. Wilde, J.J.M. Jans, J. van der Velden, R.A. de Boer, J.P. van Tintelen, F.W. Asselbergs and A.F. Baas declare that they have no competing interests.
Figures

References
-
- Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2733–2779. doi: 10.1093/eurheartj/ehu199. - DOI - PubMed
-
- Maron BJ, Gardin JM, Flack JM, et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA study. Coronary artery risk development in (young) adults. Circulation. 1995;92:785–789. doi: 10.1161/01.CIR.92.4.785. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

