Morphological and biochemical changes in the pancreas associated with acute systemic hypoxia
- PMID: 33532907
- PMCID: PMC7900369
- DOI: 10.1007/s13577-020-00481-0
Morphological and biochemical changes in the pancreas associated with acute systemic hypoxia
Abstract
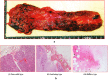
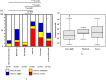
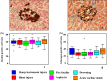
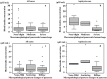
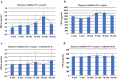
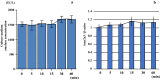
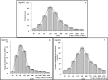
This study aimed to investigate the changes associated with acute systemic hypoxia in the endocrine system, particularly in pancreatic tissues. The investigation was based on macroscopic, pathohistological, biochemical, and molecular biological findings in cell lines and human cadavers. The results showed that cases of death due to asphyxia more frequently showed severe subcapsular/interstitial hemorrhage versus the other causes of death. Histological examination showed that asphyxia cases were associated with severe morphological changes. Although measured insulin levels in the asphyxia were higher compared to other causes of death, no differences were noted for the glucagon and amylase levels with regard to the cause of death. Increased blood insulin levels were not associated with macro- and micromorphological changes, and did not show any association with glucose or cortisol levels. The experiment conducted under hypoxic conditions in cultured cells demonstrated that insulin mRNA expression and insulin protein levels peaked at 10 min after hypoxia exposure. However, there were no changes in either the amylase mRNA or protein levels. Corticosterone level peaked at 120 min after exposure to hypoxic conditions. Overall, acute systemic hypoxic conditions can directly affect the mechanisms involved in pancreatic insulin secretion.
Keywords: Cell culture; Cortisol; Glucose; Hypoxia; Insulin.
Conflict of interest statement
The authors declare they have no conflict of interest.
Figures







References
-
- Fisher DA. Endocrinology of fatal development. In: Kronenberg HM, Melmed S, Polonsky KS, Larsen PR, editors. Williams textbook of endocrinology. 11. Philadelphia, PA: Saunders; 2007. pp. 755–782.
-
- Fink G, Pfaff DW, Levine JE, editors. Handbook of Neuroendocrinology. 1. Cambridge (MA): Academic Press; 2011.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

