Improved in vivo PET imaging of the adenosine A2A receptor in the brain using [18F]FLUDA, a deuterated radiotracer with high metabolic stability
- PMID: 33532910
- PMCID: PMC8263428
- DOI: 10.1007/s00259-020-05164-4
Improved in vivo PET imaging of the adenosine A2A receptor in the brain using [18F]FLUDA, a deuterated radiotracer with high metabolic stability
Abstract
Purpose: The adenosine A2A receptor has emerged as a therapeutic target for multiple diseases, and thus the non-invasive imaging of the expression or occupancy of the A2A receptor has potential to contribute to diagnosis and drug development. We aimed at the development of a metabolically stable A2A receptor radiotracer and report herein the preclinical evaluation of [18F]FLUDA, a deuterated isotopologue of [18F]FESCH.
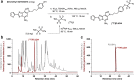
Methods: [18F]FLUDA was synthesized by a two-step one-pot approach and evaluated in vitro by autoradiographic studies as well as in vivo by metabolism and dynamic PET/MRI studies in mice and piglets under baseline and blocking conditions. A single-dose toxicity study was performed in rats.
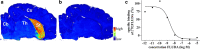
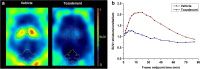
Results: [18F]FLUDA was obtained with a radiochemical yield of 19% and molar activities of 72-180 GBq/μmol. Autoradiography proved A2A receptor-specific accumulation of [18F]FLUDA in the striatum of a mouse and pig brain. In vivo evaluation in mice revealed improved stability of [18F]FLUDA compared to that of [18F]FESCH, resulting in the absence of brain-penetrant radiometabolites. Furthermore, the radiometabolites detected in piglets are expected to have a low tendency for brain penetration. PET/MRI studies confirmed high specific binding of [18F]FLUDA towards striatal A2A receptor with a maximum specific-to-non-specific binding ratio in mice of 8.3. The toxicity study revealed no adverse effects of FLUDA up to 30 μg/kg, ~ 4000-fold the dose applied in human PET studies using [18F]FLUDA.
Conclusions: The new radiotracer [18F]FLUDA is suitable to detect the availability of the A2A receptor in the brain with high target specificity. It is regarded ready for human application.
Keywords: A2A receptor; Adenosine receptors; FESCH; Fluorine-18; Neurodegeneration; Positron-emission tomography.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

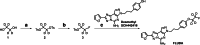



Similar articles
-
Automated radiosynthesis of the adenosine A2A receptor-targeting radiotracer [18 F]FLUDA.J Labelled Comp Radiopharm. 2022 May 30;65(6):162-166. doi: 10.1002/jlcr.3970. Epub 2022 Mar 24. J Labelled Comp Radiopharm. 2022. PMID: 35288969
-
Non-Invasive Assessment of Locally Overexpressed Human Adenosine 2A Receptors in the Heart of Transgenic Mice.Int J Mol Sci. 2022 Jan 18;23(3):1025. doi: 10.3390/ijms23031025. Int J Mol Sci. 2022. PMID: 35162950 Free PMC article.
-
Preclinical Evaluation and Quantification of 18F-Fluoroethyl and 18F-Fluoropropyl Analogs of SCH442416 as Radioligands for PET Imaging of the Adenosine A2A Receptor in Rat Brain.J Nucl Med. 2017 Mar;58(3):466-472. doi: 10.2967/jnumed.116.178103. Epub 2016 Oct 27. J Nucl Med. 2017. PMID: 27789720
-
In Vivo PET Imaging of Adenosine 2A Receptors in Neuroinflammatory and Neurodegenerative Disease.Contrast Media Mol Imaging. 2017 Nov 20;2017:6975841. doi: 10.1155/2017/6975841. eCollection 2017. Contrast Media Mol Imaging. 2017. PMID: 29348737 Free PMC article. Review.
-
Potential Therapeutic Applications of Adenosine A2A Receptor Ligands and Opportunities for A2A Receptor Imaging.Med Res Rev. 2018 Jan;38(1):5-56. doi: 10.1002/med.21432. Epub 2017 Jan 27. Med Res Rev. 2018. PMID: 28128443 Review.
Cited by
-
Development and evaluation of deuterated [18F]JHU94620 isotopologues for the non-invasive assessment of the cannabinoid type 2 receptor in brain.EJNMMI Radiopharm Chem. 2024 Dec 23;9(1):91. doi: 10.1186/s41181-024-00319-2. EJNMMI Radiopharm Chem. 2024. PMID: 39714717 Free PMC article.
-
Divergent Effects of the Nonselective Adenosine Receptor Antagonist Caffeine in Pre-Manifest and Motor-Manifest Huntington's Disease.Biomedicines. 2022 May 27;10(6):1258. doi: 10.3390/biomedicines10061258. Biomedicines. 2022. PMID: 35740281 Free PMC article.
-
Development of 18F-Labeled Radiotracers for PET Imaging of the Adenosine A2A Receptor: Synthesis, Radiolabeling and Preliminary Biological Evaluation.Int J Mol Sci. 2021 Feb 25;22(5):2285. doi: 10.3390/ijms22052285. Int J Mol Sci. 2021. PMID: 33669003 Free PMC article.
-
Quantitation of the A2A Adenosine Receptor Density in the Striatum of Mice and Pigs with [18F]FLUDA by Positron Emission Tomography.Pharmaceuticals (Basel). 2022 Apr 22;15(5):516. doi: 10.3390/ph15050516. Pharmaceuticals (Basel). 2022. PMID: 35631343 Free PMC article.
-
Metabolic Stability of the Demyelination Positron Emission Tomography Tracer [18F]3-Fluoro-4-Aminopyridine and Identification of Its Metabolites.J Pharmacol Exp Ther. 2023 Jul;386(1):93-101. doi: 10.1124/jpet.122.001462. Epub 2023 Apr 6. J Pharmacol Exp Ther. 2023. PMID: 37024145 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

