Adverse Events of Thiopurine Therapy in Pediatric Inflammatory Bowel Disease and Correlations with Metabolites: A Cohort Study
- PMID: 33532972
- PMCID: PMC8741678
- DOI: 10.1007/s10620-021-06836-3
Adverse Events of Thiopurine Therapy in Pediatric Inflammatory Bowel Disease and Correlations with Metabolites: A Cohort Study
Abstract
Background: In the recent era of growing availability of biological agents, the role of thiopurines needs to be reassessed with the focus on toxicity.
Aims: We assessed the incidence and predictive factors of thiopurine-induced adverse events (AE) resulting in therapy cessation in pediatric inflammatory bowel disease (IBD), related to thiopurine metabolites and biochemical abnormalities, and determined overall drug survival.
Methods: We performed a retrospective, single-center study of children diagnosed with IBD between 2000 and 2019 and treated with thiopurine therapy. The incidence of AE and overall drug survival of thiopurines were evaluated using the Kaplan-Meier method. Correlations between thiopurine metabolites and biochemical tests were computed using Spearman's correlation coefficient.
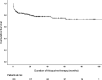
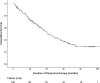
Results: Of 391 patients with IBD, 233 patients (162 Crohn's disease, 62 ulcerative colitis, and 9 IBD-unclassified) were prescribed thiopurines (230 azathioprine and 3 mercaptopurine), of whom 50 patients (22%) discontinued treatment, at least temporary, due to thiopurine-induced AE (median follow-up 20.7 months). Twenty-six patients (52%) were rechallenged and 18 of them (70%) tolerated this. Sixteen patients (6%) switched to a second thiopurine agent after azathioprine intolerance and 10 of them (63%) tolerated this. No predictive factors for development of AE could be identified. Concentrations of 6-thioguanine nucleotides (6-TGN) were significantly correlated with white blood cell and neutrophil count, 6-methylmercaptopurine (6-MMP) concentrations with alanine aminotransferase and gamma-glutamyltranspeptidase.
Conclusions: Approximately 20% of pediatric patients with IBD discontinued thiopurine treatment due to AE. A rechallenge or switch to mercaptopurine is an effective strategy after development of AE. Concentrations of 6-TGN and 6-MMP are associated with biochemical abnormalities.
Keywords: Adverse events; Inflammatory bowel disease; Metabolites; Pediatrics; Thiopurines.
© 2021. The Author(s).
Conflict of interest statement
NdB has served as a speaker for AbbVie and MSD and has served as consultant and principal investigator for TEVA Pharma BV and Takeda. He has received a (unrestricted) research grant from Dr. Falk, TEVA Pharma BV, MLDS and Takeda. JvL reports consulting, travel and/or speaker fees and research support from AbbVie, Janssen, Nestlé Health Science, Novalac, Pfizer, Merck, P&G, GSK, Illumina, Otsuka. JJ, CP, HB, MvW, MB, and TdM have nothing to declare.
Figures
References
-
- Turner D, Ruemmele FM, Orlanski-Meyer E, et al. Management of Paediatric Ulcerative Colitis, Part 1: Ambulatory Care-An Evidence-based Guideline From European Crohn's and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;67:257–291. doi: 10.1097/mpg.0000000000002035. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

