Molecular Features of Cephalosporins Important for Activity against Antimicrobial-Resistant Neisseria gonorrhoeae
- PMID: 33533239
- PMCID: PMC9847585
- DOI: 10.1021/acsinfecdis.0c00400
Molecular Features of Cephalosporins Important for Activity against Antimicrobial-Resistant Neisseria gonorrhoeae
Abstract
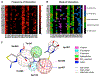
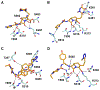
The increasing prevalence of Neisseria gonorrhoeae strains exhibiting decreased susceptibility to extended-spectrum cephalosporins (ESCs) presents a challenge for the successful treatment of gonorrhea infections. To address this challenge, we evaluated a panel of 23 cephalosporins against penicillin-binding protein 2 (PBP2) from the ESC-resistant (ESCR) N. gonorrhoeae strain H041 to determine which molecular features are important for antimicrobial activity. Structure-activity relationships (SARs) developed from acylation rate constants against PBP2 and antimicrobial susceptibilities against the H041 strain of N. gonorrhoeae, and interpreted against docking models, reveal that cephalosporins possessing large, lipophilic R1 side chains and electronegative R2 side chains with planar groups are associated with higher acylation rates against PBP2, but also that these same amphipathic features can lower antimicrobial activity. Based on these studies, we tested cefoperazone, one of the most effective ESCs for targeting PBP2, in the female mouse model infected with H041 and showed that it was equally or more effective than ceftriaxone or gentamicin for clearing infections. Taken together, our results reveal that two U.S. Food and Drug Administration (FDA)-approved agents (cefoperazone, ceftaroline) and one FDA-qualified infectious disease product (ceftobiprole) have potential as first-line treatments for gonorrhea and provide a framework for the future design of cephalosporins with improved activity against ESC-resistant N. gonorrhoeae.
Keywords: Neisseria gonorrhoeae; cephalosporins; molecular docking; penicillin-binding protein 2; structure−activity relationship.
Figures
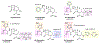
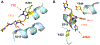
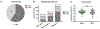
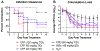
References
-
- Satterwhite CL; Torrone E; Meites E; Dunne EF; Mahajan R; Ocfemia MC; Su J; Xu F; Weinstock H, Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis 2013, 40, 187–193. - PubMed
-
- Newman L; Rowley J; Vander Hoorn S; Wijesooriya NS; Unemo M; Low N; Stevens G; Gottlieb S; Kiarie J; Temmerman M, Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS One 2015, 10, e0143304. - PMC - PubMed
-
- Wasserheit JN, Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex. Transm. Dis 1992, 19, 61–77. - PubMed
-
- Akasaka S; Muratani T; Yamada Y; Inatomi H; Takahashi K; Matsumoto T, Emergence of cephem- and aztreonam-high-resistant Neisseria gonorrhoeae that does not produce beta-lactamase. J. Infect. Chemother 2001, 7, 49–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

