Early response by MR imaging and ultrasound as predictor of pathologic complete response to 12-week neoadjuvant therapy for different early breast cancer subtypes: Combined analysis from the WSG ADAPT subtrials
- PMID: 33533487
- PMCID: PMC8048810
- DOI: 10.1002/ijc.33495
Early response by MR imaging and ultrasound as predictor of pathologic complete response to 12-week neoadjuvant therapy for different early breast cancer subtypes: Combined analysis from the WSG ADAPT subtrials
Abstract
We evaluated the role of early response after 3 weeks of neoadjuvant treatment (NAT) assessed by ultrasound (US), magnetic resonance imaging (MRI) and Ki-67 dynamics for prediction of pathologic complete response (pCR) in different early breast cancer subtypes. Patients with HR+/HER2+, HR-/HER2- and HR-/HER2+ tumors enrolled into three neoadjuvant WSG ADAPT subtrials underwent US, MRI and Ki-67 assessment at diagnosis and after 3 weeks of NAT. Early response was defined as complete or partial response (US, MRI) and ≥30% proliferation decrease or <500 invasive tumor cells (Ki-67). Predictive values and area under the receiver operating characteristic (AUC) curves for prediction of pCR (ypT0/is ypN0) after 12-week NAT were calculated. Two hundred twenty-six had MRI and 401 US; 107 underwent both MRI and US. All three methods yielded a similar AUC in HR+/HER2+ (0.66-0.67) and HR-/HER2- tumors (0.53-0.63), while MRI and Ki-67 performed better than US in HR-/HER2+ tumors (0.83 and 0.79 vs 0.56). Adding MRI+/-Ki-67 increased AUC of US in HR-/HER2+ tumors to 0.64 to 0.75. MRI and Ki-67 demonstrated highest sensitivity in HR-/HER2- (0.8-1) and HR-/HER2+ tumors (1, both). Negative predictive value was similar for all methods in HR+/HER2+ (0.71-0.74) and HR-/HER2- tumors (0.85-1), while it was higher for MRI and Ki-67 compared to US in HR-/HER2+ subtype (1 vs 0.5). Early response assessed by US, MRI and Ki-67 is a strong predictor for pCR after 12-week NAT. Strength of pCR prediction varies according to tumor subtype. Adding MRI+/-Ki-67 to US did not improve pCR prediction in majority of our patients.
Keywords: breast cancer; magnetic resonance imaging; neoadjuvant therapy; pathologic complete response; ultrasound.
© 2021 Westdeutsche Studiengruppe GmbH. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of Union for International Cancer Control.
Conflict of interest statement
Oleg Gluz acts as Co‐Director West German Study Group (WSG), received honoraria from Genomic Health/Exact Sciences, Roche, Celgene, Pfizer, Novartis, NanoString Technologies, AstraZeneca, served in consulting/advisory role for Celgene, Genomic Health/Exact Sciences, Lilly, MSD, Novartis, Pfizer, Roche, and received travel support from Roche. Rachel Würstlein served in consulting/advisory role as well as on speakers' bureau for and received travel support from Agendia, Amgen, Aristo, AstraZeneca, Boeringer Ingelheim, Carl Zeiss, Celgene, Clinsol, Daiichi‐Sankyo, Eisai, Genomic Health, Glaxo Smith Kline, Hexal, Lilly, Medstrom Medical, MSD, Mundipharma, Nanostring, Novartis, Odonate, Onkowissen, Paxman, Palleos, Pfizer, Pierre Fabre, PumaBiotechnolgogy, Riemser, Roche, Sandoz/Hexal, Seattle Genetics, Tesaro Bio, Teva, Viatris. Sherko Kümmel acts as Co‐Director West German Study Group (WSG), received personal fees from Lilly, Roche, Genomic Health, Novartis, Amgen, Celgene, Daiichi Sankyo, AstraZeneca, SOMATEX Medical Technologies, MSD, Pfizer, Puma Biotechnology, PFM medical, and nonfinancial support from Roche, Daiichi Sankyo, Sonoscope. Michael Braun received honoraria from AstraZeneca, Exact Sciences, Novartis, Pfizer, Roche, Teva, travel support from AstraZeneca, Celgene, Medac, Novartis, Roche and served in consulting/advisory role for AstraZeneca, Exact Sciences, Novartis, Puma, Roche. Bahriye Aktas reports a potential financial conflict of interest as follows: Pfizer Pharma GmbH, Roche Pharma AG, Novartis Pharma GmbH, AstraZeneca GmbH, PharmaMar GmbH, MSD Merck Sharp & Dohme GmbH, Onkowissen.de GmbH, Lilly Deutschland GmbH, Promedicis GmbH. Cornelia Kolberg‐Liedtke has ownership interest in Theraklion, Phaon Scientific (both applicable for immediate family member), received honoraria from Roche, AstraZeneca, Celgene, Novartis, Pfizer, Lilly, Hexal, Amgen, SonoScape, and an immediate family member received honoraria from Pfizer, Novartis, Roche, Genomic Health, Amgen, AstraZeneca, Riemser, Carl Zeiss MediTec, TEVA Pharmaceuticals Industries, Theraklion, Janssen‐Cilag, GlaxoSmithKline, LIV Pharma, served in consulting/advisory role for Roche, Novartis, Pfizer, Celgene, Phaon Scientific, and an immediate family member served in consulting/advisory role for Pfizer, Novartis, SurgVision, CarlZeissMeditec, Amgen, Onkowissen, received research funding from Roche, Novartis, Pfizer, and received travel support from Roche, Daiichi Sankyo, Novartis, and an immediate family member received research funding from Carl Zeiss Meditec, LIV Pharma, Novartis, Amgen, Pfizer, Daiichi Sankyo. Nadia Harbeck acts as Co‐Director West German Study Group (WSG) and reports a potential financial conflict of interest as follows: AstraZeneca, Lilly, Novartis, Pfizer, Pierre Fabre, Roche/Genentech, Merck Sharp & Dohme, Seattle Genetics. Ulrike Nitz acts as Co‐Director West German Study Group (WSG), received honoraria from Agendia, Amgen, Celgene, Genomic Health, NanoString Technologies, Novartis pharma, Pfizer Pharmaceuticals, Roche/Genentech, Teva, served in consulting/advisory role for Genomic Health, Roche, provided expert testimony for Genomic Health, received travel support from Genomic Health, Pfizer Pharmaceuticals, Roche, and her institution received research funding from Agendia, Amgen, Celgene, Genomic Health, NanoString Technologies, Roche, Sanofi.
Figures
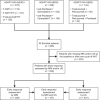

References
-
- Arnaout A, Lee J, Gelmon K, et al. Neoadjuvant therapy for breast cancer: updates and proceedings from the Seventh Annual Meeting of the Canadian Consortium for Locally Advanced Breast Cancer. Curr Oncol. 2018;25:e490‐e498.
-
- Fowler AM, Mankoff DA, Joe BN. Imaging neoadjuvant therapy response in breast cancer. Radiology. 2017;285:358‐375. - PubMed
-
- Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta‐analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48:3342‐3354. - PubMed
-
- von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796‐1804. - PubMed
-
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long‐term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164‐172. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

