Teaching Aldehydes New Tricks Using Rhodium- and Cobalt-Hydride Catalysis
- PMID: 33533586
- PMCID: PMC8486976
- DOI: 10.1021/acs.accounts.0c00771
Teaching Aldehydes New Tricks Using Rhodium- and Cobalt-Hydride Catalysis
Abstract
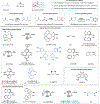
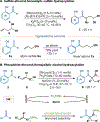
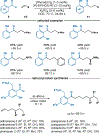
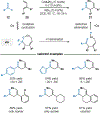
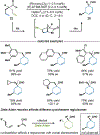
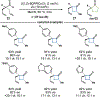
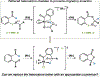
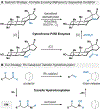
By using transition metal catalysts, chemists have altered the "logic of chemical synthesis" by enabling the functionalization of carbon-hydrogen bonds, which have traditionally been considered inert. Within this framework, our laboratory has been fascinated by the potential for aldehyde C-H bond activation. Our approach focused on generating acyl-metal-hydrides by oxidative addition of the formyl C-H bond, which is an elementary step first validated by Tsuji in 1965. In this Account, we review our efforts to overcome limitations in hydroacylation. Initial studies resulted in new variants of hydroacylation and ultimately spurred the development of related transformations (e.g., carboacylation, cycloisomerization, and transfer hydroformylation).Sakai and co-workers demonstrated the first hydroacylation of olefins when they reported that 4-pentenals cyclized to cyclopentanones, using stoichiometric amounts of Wilkinson's catalyst. This discovery sparked significant interest in hydroacylation, especially for the enantioselective and catalytic construction of cyclopentanones. Our research focused on expanding the asymmetric variants to access medium-sized rings (e.g., seven- and eight-membered rings). In addition, we achieved selective intermolecular couplings by incorporating directing groups onto the olefin partner. Along the way, we identified Rh and Co catalysts that transform dienyl aldehydes into a variety of unique carbocycles, such as cyclopentanones, bicyclic ketones, cyclohexenyl aldehydes, and cyclobutanones. Building on the insights gained from olefin hydroacylation, we demonstrated the first highly enantioselective hydroacylation of carbonyls. For example, we demonstrated that ketoaldehydes can cyclize to form lactones with high regio- and enantioselectivity. Following these reports, we reported the first intermolecular example that occurs with high stereocontrol. Ketoamides undergo intermolecular carbonyl hydroacylation to furnish α-acyloxyamides that contain a depsipeptide linkage.Finally, we describe how the key acyl-metal-hydride species can be diverted to achieve a C-C bond-cleaving process. Transfer hydroformylation enables the preparation of olefins from aldehydes by a dehomologation mechanism. Release of ring strain in the olefin acceptor offers a driving force for the isodesmic transfer of CO and H2. Mechanistic studies suggest that the counterion serves as a proton-shuttle to enable transfer hydroformylation. Collectively, our studies showcase how transition metal catalysis can transform a common functional group, in this case aldehydes, into structurally distinct motifs. Fine-tuning the coordination sphere of an acyl-metal-hydride species can promote C-C and C-O bond-forming reactions, as well as C-C bond-cleaving processes.
Figures

















References
-
- von Delius M; Le CM; Dong VM Rhodium-Phosphoramidite Catalyzed Alkene Hydroacylation: Mechanism and Octaketide Natural Product Synthesis. J. Am. Chem. Soc 2012, 134, 15022–15032. - PubMed
-
- Shen Z; Khan HA; Dong VM Rh-Catalyzed Carbonyl Hydroacylation: An Enantioselective Approach to Lactones. J. Am. Chem. Soc 2008, 130, 2916–2917. - PubMed
-
- Franke R; Selent D; Börner A. Applied Hydroformylation. Chem. Rev 2012, 112, 5675–5732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

