SARS-CoV-2 induces human plasmacytoid predendritic cell diversification via UNC93B and IRAK4
- PMID: 33533916
- PMCID: PMC7849819
- DOI: 10.1084/jem.20201387
SARS-CoV-2 induces human plasmacytoid predendritic cell diversification via UNC93B and IRAK4
Abstract
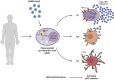
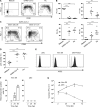
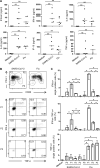
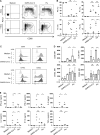
Several studies have analyzed antiviral immune pathways in late-stage severe COVID-19. However, the initial steps of SARS-CoV-2 antiviral immunity are poorly understood. Here we have isolated primary SARS-CoV-2 viral strains and studied their interaction with human plasmacytoid predendritic cells (pDCs), a key player in antiviral immunity. We show that pDCs are not productively infected by SARS-CoV-2. However, they efficiently diversified into activated P1-, P2-, and P3-pDC effector subsets in response to viral stimulation. They expressed CD80, CD86, CCR7, and OX40 ligand at levels similar to influenza virus-induced activation. They rapidly produced high levels of interferon-α, interferon-λ1, IL-6, IP-10, and IL-8. All major aspects of SARS-CoV-2-induced pDC activation were inhibited by hydroxychloroquine. Mechanistically, SARS-CoV-2-induced pDC activation critically depended on IRAK4 and UNC93B1, as established using pDC from genetically deficient patients. Overall, our data indicate that human pDC are efficiently activated by SARS-CoV-2 particles and may thus contribute to type I IFN-dependent immunity against SARS-CoV-2 infection.
© 2021 Onodi et al.
Conflict of interest statement
Disclosures: J.-M. Molina reported grants from Gilead and personal fees from Merck, ViiV, and Janssen outside the submitted work. V. Soumelis reported grants from Sanofi and Roche, and personal fees from Leo Pharma, Gilead, Merck, and Sanofi outside the submitted work. No other disclosures were reported.
Figures




Update of
-
SARS-CoV-2 induces human plasmacytoid pre-dendritic cell diversification via UNC93B and IRAK4.bioRxiv [Preprint]. 2021 Jan 8:2020.07.10.197343. doi: 10.1101/2020.07.10.197343. bioRxiv. 2021. Update in: J Exp Med. 2021 Apr 5;218(4):e20201387. doi: 10.1084/jem.20201387. PMID: 33442685 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

