Methylprednisolone in adults hospitalized with COVID-19 pneumonia : An open-label randomized trial (GLUCOCOVID)
- PMID: 33534047
- PMCID: PMC7854876
- DOI: 10.1007/s00508-020-01805-8
Methylprednisolone in adults hospitalized with COVID-19 pneumonia : An open-label randomized trial (GLUCOCOVID)
Abstract
Purpose: To determine whether a 6-day course of methylprednisolone (MP) improves outcome in patients with severe SARS-CoV‑2 (Corona Virus Disease 2019 [COVID-19]).
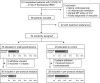
Methods: The study was a multicentric open-label trial of COVID-19 patients who were aged ≥ 18 years, receiving oxygen without mechanical ventilation, and with evidence of systemic inflammatory response who were assigned to standard of care (SOC) or SOC plus intravenous MP (40 mg bid for 3 days followed by 20 mg bid for 3 days). The primary outcome was a composite of death, admission to the intensive care unit, or requirement for noninvasive ventilation. Both intention-to-treat (ITT) and per protocol (PP) analyses were performed.
Results: A total of 91 patients were screened, and 64 were randomized (mean age70 ± 12 years). In the ITT analysis, 14 of 29 patients (48%) in the SOC group and 14 of 35 (40%) in the MP group suffered the composite endpoint (40% versus 20% in patients under 72 years and 67% versus 48% in those over 72 years; p = 0.25). In the PP analysis, patients on MP had a significantly lower risk of experiencing the composite endpoint (age-adjusted risk ratio 0.42; 95% confidence interval, CI 0.20-0.89; p = 0.043).
Conclusion: The planned sample size was not achieved, and our results should therefore be interpreted with caution. The use of MP had no significant effect on the primary endpoint in ITT analysis; however, the PP analysis showed a beneficial effect due to MP, which consistent with other published trials support the use of glucocorticoids in severe cases of COVID-19.
Keywords: Coronavirus Infections; Glucocorticoids; Humans; Mortality; SARS-CoV-2.
Conflict of interest statement
L. Corral-Gudino, A. Bahamonde, F. Arnaiz-Revillas, J. Gómez-Barquero, J. Abadía-Otero, C. García-Ibarbia, V. Mora, A. Cerezo-Hernández, J.L. Hernández, G. López-Muñíz, F. Hernández-Blanco, J.M. Cifrián, J.M. Olmos, M. Carrascosa, L. Nieto, M.C. Fariñas, J.A. Riancho, and GLUCOCOVID investigators declare that they have no competing interests.
Figures


References
-
- Clinical management of severe acute respiratory infection when COVID-19 is suspected [.. https://www.who.int/publications-detail/clinical-management-of-severe-ac.... Accessed 2 Apr 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

