Rearrangement of Actin Cytoskeleton by Zika Virus Infection Facilitates Blood-Testis Barrier Hyperpermeability
- PMID: 33534087
- PMCID: PMC8379325
- DOI: 10.1007/s12250-020-00343-x
Rearrangement of Actin Cytoskeleton by Zika Virus Infection Facilitates Blood-Testis Barrier Hyperpermeability
Abstract
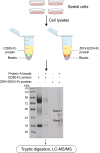
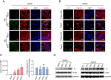
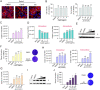
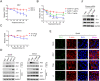
In recent years, various serious diseases caused by Zika virus (ZIKV) have made it impossible to be ignored. Confirmed existence of ZIKV in semen and sexually transmission of ZIKV suggested that it can break the blood-testis barrier (BTB), or Sertoli cell barrier (SCB). However, little is known about the underlying mechanism. In this study, interaction between actin, an important component of the SCB, and ZIKV envelope (E) protein domain III (EDIII) was inferred from co-immunoprecipitation (Co-IP) liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. Confocal microscopy confirmed the role of actin filaments (F-actin) in ZIKV infection, during which part of the stress fibers, the bundles that constituted by paralleled actin filaments, were disrupted and presented in the cell periphery. Colocalization of E and reorganized actin filaments in the cell periphery of transfected Sertoli cells suggests a participation of ZIKV E protein in ZIKV-induced F-actin rearrangement. Perturbation of F-actin by cytochalasin D (CytoD) or Jasplakinolide (Jas) enhanced the infection of ZIKV. More importantly, the transepithelial electrical resistance (TEER) of an in vitro mouse SCB (mSCB) model declined with the progression of ZIKV infection or overexpression of E protein. Co-IP and confocal microscopy analyses revealed that the interaction between F-actin and tight junction protein ZO-1 was reduced after ZIKV infection or E protein overexpression, highlighting the role of E protein in ZIKV-induced disruption of the BTB. We conclude that the interaction between ZIKV E and F-actin leads to the reorganization of F-actin network, thereby compromising BTB integrity.
Keywords: Actin filaments; Blood-testis barrier (BTB); Envelope protein; Sertoli cell barrier (SCB); Zika virus (ZIKV).
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Zika Virus Infects Human Sertoli Cells and Modulates the Integrity of the In Vitro Blood-Testis Barrier Model.J Virol. 2017 Oct 27;91(22):e00623-17. doi: 10.1128/JVI.00623-17. Print 2017 Nov 15. J Virol. 2017. PMID: 28878076 Free PMC article.
-
Matrix metalloproteinase 9 facilitates Zika virus invasion of the testis by modulating the integrity of the blood-testis barrier.PLoS Pathog. 2020 Apr 17;16(4):e1008509. doi: 10.1371/journal.ppat.1008509. eCollection 2020 Apr. PLoS Pathog. 2020. PMID: 32302362 Free PMC article.
-
Sertoli Cells Are Susceptible to ZIKV Infection in Mouse Testis.Front Cell Infect Microbiol. 2017 Jun 21;7:272. doi: 10.3389/fcimb.2017.00272. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28680856 Free PMC article.
-
Zika virus and reproduction: facts, questions and current management.Hum Reprod Update. 2017 Nov 1;23(6):629-645. doi: 10.1093/humupd/dmx024. Hum Reprod Update. 2017. PMID: 28961800 Review.
-
Regulation of Blood-Testis Barrier (BTB) Dynamics, Role of Actin-, and Microtubule-Based Cytoskeletons.Methods Mol Biol. 2018;1748:229-243. doi: 10.1007/978-1-4939-7698-0_16. Methods Mol Biol. 2018. PMID: 29453575 Free PMC article. Review.
Cited by
-
Dengue Virus Infection Alters Inter-Endothelial Junctions and Promotes Endothelial-Mesenchymal-Transition-Like Changes in Human Microvascular Endothelial Cells.Viruses. 2023 Jun 26;15(7):1437. doi: 10.3390/v15071437. Viruses. 2023. PMID: 37515125 Free PMC article.
-
In vitro and in vivo efficacy of Molnupiravir against Zika virus infections.Virol Sin. 2023 Aug;38(4):639-642. doi: 10.1016/j.virs.2023.05.011. Epub 2023 Jun 1. Virol Sin. 2023. PMID: 37268161 Free PMC article.
-
Zika virus NS1 drives tunneling nanotube formation for mitochondrial transfer and stealth transmission in trophoblasts.Nat Commun. 2025 Feb 20;16(1):1803. doi: 10.1038/s41467-025-56927-2. Nat Commun. 2025. PMID: 39979240 Free PMC article.
-
Zika virus disrupts steroidogenesis and impairs spermatogenesis by stalling the translation of CYP17A1 mRNA.Nat Commun. 2025 Jul 22;16(1):6756. doi: 10.1038/s41467-025-62044-x. Nat Commun. 2025. PMID: 40695837 Free PMC article.
-
ROCK1/MLC2 inhibition induces decay of viral mRNA in BPXV infected cells.Sci Rep. 2022 Oct 24;12(1):17811. doi: 10.1038/s41598-022-21610-9. Sci Rep. 2022. PMID: 36280692 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

