Sensitivity of anti-SARS-CoV-2 serological assays in a high-prevalence setting
- PMID: 33534090
- PMCID: PMC7856849
- DOI: 10.1007/s10096-021-04169-7
Sensitivity of anti-SARS-CoV-2 serological assays in a high-prevalence setting
Abstract
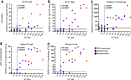
Evaluation and power of seroprevalence studies depend on the performed serological assays. The aim of this study was to assess four commercial serological tests from EUROIMMUN, DiaSorin, Abbott, and Roche as well as an in-house immunofluorescence and neutralization test for their capability to identify SARS-CoV-2 seropositive individuals in a high-prevalence setting. Therefore, 42 social and working contacts of a German super-spreader were tested. Consistent with a high-prevalence setting, 26 of 42 were SARS-CoV-2 seropositive by neutralization test (NT), and immunofluorescence test (IFT) confirmed 23 of these 26 positive test results (NT 61.9% and IFT 54.8% seroprevalence). Four commercial assays detected anti-SARS-CoV-2 antibodies in 33.3-40.5% individuals. Besides an overall discrepancy between the NT and the commercial assays regarding their sensitivity, this study revealed that commercial SARS-CoV-2 spike-based assays are better to predict the neutralization titer than nucleoprotein-based assays are.
Keywords: COVID-19; Immunofluorescence test; Neutralizing antibodies; SARS-CoV-2; Serology; Seroprevalence.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, Chen H-D, Chen J, Luo Y, Guo H, Jiang R-D, Liu M-Q, Chen Y, Shen X-R, Wang X, Zheng X-S, Zhao K, Chen Q-J, Deng F, Liu L-L, Yan B, Zhan F-X, Wang Y-Y, Xiao G-F, Shi Z-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
-
- Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, Haagmans BL, Lauber C, Leontovich AM, Neuman BW, Penzar D, Perlman S, LLM P, Samborskiy DV, Sidorov IA, Sola I, Ziebuhr J, Coronaviridae Study Group of the International Committee on Taxonomy of V The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. - DOI - PMC - PubMed
-
- COVID 19 Pandemic Emergency Information (2020) https://covid19.who.int/
-
- Whitman JD, Hiatt J, Mowery CT, Shy BR, Yu R, Yamamoto TN, Rathore U, Goldgof GM, Whitty C, Woo JM, Gallman AE, Miller TE, Levine AG, Nguyen DN, Bapat SP, Balcerek J, Bylsma SA, Lyons AM, Li S, Wong AW, Gillis-Buck EM, Steinhart ZB, Lee Y, Apathy R, Lipke MJ, Smith JA, Zheng T, Boothby IC, Isaza E, Chan J, Acenas DD 2nd, Lee J, Macrae TA, Kyaw TS, Wu D, Ng DL, Gu W, York VA, Eskandarian HA, Callaway PC, Warrier L, Moreno ME, Levan J, Torres L, Farrington LA, Loudermilk RP, Koshal K, Zorn KC, Garcia-Beltran WF, Yang D, Astudillo MG, Bernstein BE, Gelfand JA, Ryan ET, Charles RC, Iafrate AJ, Lennerz JK, Miller S, Chiu CY, Stramer SL, Wilson MR, Manglik A, Ye CJ, Krogan NJ, Anderson MS, Cyster JG, Ernst JD, AHB W, Lynch KL, Bern C, Hsu PD, Marson A (2020) Evaluation of SARS-CoV-2 serology assays reveals a range of test performance. Nat Biotechnol. 10.1038/s41587-020-0659-0
-
- Krammer F, Simon V (2020) Serology assays to manage COVID-19. Science eabc1227. 10.1126/science.abc1227 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

