Group 2 Innate Lymphoid Cells: Team Players in Regulating Asthma
- PMID: 33534604
- PMCID: PMC7614118
- DOI: 10.1146/annurev-immunol-110119-091711
Group 2 Innate Lymphoid Cells: Team Players in Regulating Asthma
Abstract
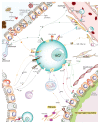
Type 2 immunity helps protect the host from infection, but it also plays key roles in tissue homeostasis, metabolism, and repair. Unfortunately, inappropriate type 2 immune reactions may lead to allergy and asthma. Group 2 innate lymphoid cells (ILC2s) in the lungs respond rapidly to local environmental cues, such as the release of epithelium-derived type 2 initiator cytokines/alarmins, producing type 2 effector cytokines such as IL-4, IL-5, and IL-13 in response to tissue damage and infection. ILC2s are associated with the severity of allergic asthma, and experimental models of lung inflammation have shown how they act as playmakers, receiving signals variously from stromal and immune cells as well as the nervous system and then distributing cytokine cues to elicit type 2 immune effector functions and potentiate CD4+ T helper cell activation, both of which characterize the pathology of allergic asthma. Recent breakthroughs identifying stromal- and neuronal-derived microenvironmental cues that regulate ILC2s, along with studies recognizing the potential plasticity of ILC2s, have improved our understanding of the immunoregulation of asthma and opened new avenues for drug discovery.
Keywords: alarmin; allergy; asthma; innate lymphoid cells; neuroimmunity; type 2 immunity.
Figures



References
-
- Croisant S. Epidemiology of asthma: prevalence and burden of disease. Adv Exp Med Biol. 2014;795:17–29. - PubMed
-
- Koczulla AR, Vogelmeier CF, Garn H, Renz H. New concepts in asthma: clinical phenotypes and pathophysiological mechanisms. Drug Discov Today. 2017;22:388–96. - PubMed
-
- Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. - PubMed
-
- Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie AN. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129:191–8.:e1-4. - PubMed
-
- Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

