The IgG3 subclass of β1-adrenergic receptor autoantibodies is an endogenous biaser of β1AR signaling
- PMID: 33534612
- PMCID: PMC8101462
- DOI: 10.1091/mbc.E20-06-0394
The IgG3 subclass of β1-adrenergic receptor autoantibodies is an endogenous biaser of β1AR signaling
Abstract
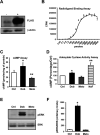
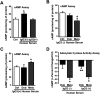
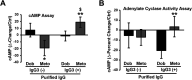
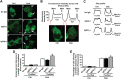
Dysregulation of immune responses has been linked to the generation of immunoglobulin G (IgG) autoantibodies that target human β1ARs and contribute to deleterious cardiac outcomes. Given the benefits of β-blockers observed in patients harboring the IgG3 subclass of autoantibodies, we investigated the role of these autoantibodies in human β1AR function. Serum and purified IgG3(+) autoantibodies from patients with onset of cardiomyopathy were tested using human embryonic kidney (HEK) 293 cells expressing human β1ARs. Unexpectedly, pretreatment of cells with IgG3(+) serum or purified IgG3(+) autoantibodies impaired dobutamine-mediated adenylate cyclase (AC) activity and cyclic adenosine monophosphate (cAMP) generation while enhancing biased β-arrestin recruitment and Extracellular Regulated Kinase (ERK) activation. In contrast, the β-blocker metoprolol increased AC activity and cAMP in the presence of IgG3(+) serum or IgG3(+) autoantibodies. Because IgG3(+) autoantibodies are specific to human β1ARs, non-failing human hearts were used as an endogenous system to determine their ability to bias β1AR signaling. Consistently, metoprolol increased AC activity, reflecting the ability of the IgG3(+) autoantibodies to bias β-blocker toward G-protein coupling. Importantly, IgG3(+) autoantibodies are specific toward β1AR as they did not alter β2AR signaling. Thus, IgG3(+) autoantibody biases β-blocker toward G-protein coupling while impairing agonist-mediated G-protein activation but promoting G-protein-independent ERK activation. This phenomenon may underlie the beneficial outcomes observed in patients harboring IgG3(+) β1AR autoantibodies.
Figures




References
-
- Baba A (2010). Targeted autoantibodies in apheresis treatment against severe heart failure. Jpn J Apheresis 29, 187–193.
-
- Bristow MR (1997). Mechanism of action of beta-blocking agents in heart failure. Am J Cardiol 80, 26L–40L. - PubMed
-
- Christ T, Wettwer E, Dobrev D, Adolph E, Knaut M, Wallukat G, Ravens U (2001). Autoantibodies against the beta1 adrenoceptor from patients with dilated cardiomyopathy prolong action potential duration and enhance contractility in isolated cardiomyocytes. J Mol Cell Cardiol 33, 1515–1525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

