Dynamics of pseudo-atrophy in RRMS reveals predominant gray matter compartmentalization
- PMID: 33534940
- PMCID: PMC7951094
- DOI: 10.1002/acn3.51302
Dynamics of pseudo-atrophy in RRMS reveals predominant gray matter compartmentalization
Abstract
Objective: To assess the dynamics of "pseudo-atrophy," the accelerated brain volume loss observed after initiation of anti-inflammatory therapies, in patients with multiple sclerosis (MS).
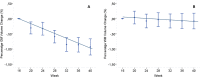
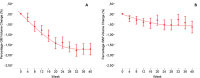
Methods: Monthly magnetic resonance imaging (MRI) data of patients from the IMPROVE clinical study (NCT00441103) comparing relapsing-remitting MS patients treated with interferon beta-1a (IFNβ-1a) for 40 weeks versus those receiving placebo (16 weeks) and then IFNβ-1a (24 weeks) were used to assess percentage of gray (PGMVC) and white matter (PWMVC) volume changes. Comparisons of PGMVC and PWMVC slopes were performed with a mixed effect linear model. In the IFNβ-1a-treated arm, a quadratic term was included in the model to evaluate the plateauing effect over 40 weeks.
Results: Up to week 16, PGMVC was -0.14% per month in the placebo and -0.27% per month in treated patients (P < 0.001). Over the same period, the decrease in PWMVC was -0.067% per month in the placebo and -0.116% per month in treated patients (P = 0.27). Similar changes were found in the group originally randomized to placebo when starting IFNβ-1a treatment (week 16-40, reliability analysis). In the originally treated group, over 40 weeks, the decrease in PGMVC showed a significant (P < 0.001) quadratic component, indicating a plateauing at week 20.
Interpretation: Findings reported here add new insights into the complex mechanisms of pseudo-atrophy and its relation to the compartmentalized inflammation occurring in the GM of MS patients. Ongoing and forthcoming clinical trials including MRI-derived GM volume loss as an outcome measure need to account for potentially significant GM volume changes as part of the initial treatment effect.
© 2021 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
NDS is a consultant for Biogen, Merck KGaA, Novartis, Roche, Sanofi‐Genzyme, Schering and Teva; has grants or grants pending from FISM and Novartis, is on the speaker bureaus of Biogen, Teva, Novartis, Sanofi‐Genzyme, Roche, and Merck KGaA; has received travel funds from Merck KGaA, Novartis, Roche, Sanofi‐Genzyme and Teva. RC was awarded 2019 MAGNIMS‐ECTRIMS fellowship. CG has received fee as speaker or advisory board by Teva, Novartis, Roche, Merck KGaA, Bayer, Almirall, Biogen. AV and AP are employees of Merck Serono S.p.A., Rome, Italy, an affiliate of Merck KGaA, Darmstadt, Germany. MPS has received consulting fees from Biogen, GeNeuro, Genzyme, MedDay, Merck KGaA, Novartis, Roche and Teva. AG, GG, MLS, and MB have nothing to disclose.
Figures

References
-
- Giorgio A, De Stefano N. Clinical use of brain volumetry. J Magn Reson Imaging 2013;37:1–14.Web of Science. - PubMed
-
- Battaglini M, Gentile G, Luchetti L, et al. Lifespan normative data on rates of brain volume changes. Neurobiol Aging 2019;81:30–37. - PubMed
-
- De Stefano N, Airas L, Grigoriadis N, et al. Clinical relevance of brain volume measures in multiple sclerosis. CNS Drugs 2014;28:147–156.Web of Science. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
