Dynamics of Neutralizing Antibody Titers in the Months After Severe Acute Respiratory Syndrome Coronavirus 2 Infection
- PMID: 33535236
- PMCID: PMC7543487
- DOI: 10.1093/infdis/jiaa618
Dynamics of Neutralizing Antibody Titers in the Months After Severe Acute Respiratory Syndrome Coronavirus 2 Infection
Abstract
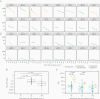
Most individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) develop neutralizing antibodies that target the viral spike protein. In this study, we quantified how levels of these antibodies change in the months after SARS-CoV-2 infection by examining longitudinal samples collected approximately 30-152 days after symptom onset from a prospective cohort of 32 recovered individuals with asymptomatic, mild, or moderate-severe disease. Neutralizing antibody titers declined an average of about 4-fold from 1 to 4 months after symptom onset. This decline in neutralizing antibody titers was accompanied by a decline in total antibodies capable of binding the viral spike protein or its receptor-binding domain. Importantly, our data are consistent with the expected early immune response to viral infection, where an initial peak in antibody levels is followed by a decline to a lower plateau. Additional studies of long-lived B cells and antibody titers over longer time frames are necessary to determine the durability of immunity to SARS-CoV-2.
Keywords: COVID-19; RBD; SARS-CoV-2; antibody dynamics; neutralizing antibodies; spike.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

References
-
- Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications.medRxiv [Preprint]. 20 April 2020. Available from: 10.1101/2020.03.30.20047365. Accessed 20 July 2020. - DOI
-
- Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 2020; 26:845–8. - PubMed
-
- Seow J, Graham C, Merrick B, et al. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection. medRxiv [Preprint]. 11 July 2020. Available from: https://www.medrxiv.org/content/10.1101/2020.07.09.20148429v1. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

