Reduced Graphene Oxide and Polyaniline Nanofibers Nanocomposite for the Development of an Amperometric Glucose Biosensor
- PMID: 33535400
- PMCID: PMC7867097
- DOI: 10.3390/s21030948
Reduced Graphene Oxide and Polyaniline Nanofibers Nanocomposite for the Development of an Amperometric Glucose Biosensor
Abstract
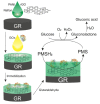
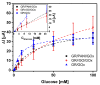
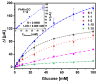
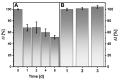
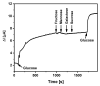
The control of glucose concentration is a crucial factor in clinical diagnosis and the food industry. Electrochemical biosensors based on reduced graphene oxide (rGO) and conducting polymers have a high potential for practical application. A novel thermal reduction protocol of graphene oxide (GO) in the presence of malonic acid was applied for the synthesis of rGO. The rGO was characterized by scanning electron microscopy, X-ray diffraction analysis, Fourier-transform infrared spectroscopy, and Raman spectroscopy. rGO in combination with polyaniline (PANI), Nafion, and glucose oxidase (GOx) was used to develop an amperometric glucose biosensor. A graphite rod (GR) electrode premodified with a dispersion of PANI nanostructures and rGO, Nafion, and GOx was proposed as the working electrode of the biosensor. The optimal ratio of PANI and rGO in the dispersion used as a matrix for GOx immobilization was equal to 1:10. The developed glucose biosensor was characterized by a wide linear range (from 0.5 to 50 mM), low limit of detection (0.089 mM), good selectivity, reproducibility, and stability. Therefore, the developed biosensor is suitable for glucose determination in human serum. The PANI nanostructure and rGO dispersion is a promising material for the construction of electrochemical glucose biosensors.
Keywords: electrochemical glucose biosensor; polyaniline nanostructures; reduced graphene oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Pires C., Duarte D. Encyclopedia of Information Science and Technology. 5th ed. IGI Global; Hershey, PA, USA: 2020. A Regulatory and Safety Perspective on Medical Devices; pp. 1815–1826.
-
- Şavk A., Aydın H., Cellat K., Şen F. A novel high performance non-enzymatic electrochemical glucose biosensor based on activated carbon-supported Pt-Ni nanocomposite. J. Mol. Liq. 2020;300:112355. doi: 10.1016/j.molliq.2019.112355. - DOI
-
- German N., Ramanavicius A., Ramanaviciene A. Amperometric Glucose Biosensor Based on Electrochemically Deposited Gold Nanoparticles Covered by Polypyrrole. Electroanalysis. 2017;29:1267–1277. doi: 10.1002/elan.201600680. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

