Investigation of Equine In Vivo and In Vitro Derived Metabolites of the Selective Androgen Receptor Modulator (SARM) ACP-105 for Improved Doping Control
- PMID: 33535528
- PMCID: PMC7912737
- DOI: 10.3390/metabo11020085
Investigation of Equine In Vivo and In Vitro Derived Metabolites of the Selective Androgen Receptor Modulator (SARM) ACP-105 for Improved Doping Control
Abstract
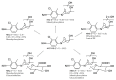
Selective Androgen Receptor Modulators (SARMs) have anabolic properties but less adverse effects than anabolic androgenic steroids. They are prohibited in both equine and human sports and there have been several cases of SARMs findings reported over the last few years. The aim of this study was to investigate the metabolite profile of the SARM ACP-105 (2-chloro-4-[(3-endo)-3-hydroxy-3-methyl-8-azabicyclo[3.2.1]oct-8-yl]-3-methylbenzonitrile) in order to find analytical targets for doping control. Oral administration of ACP-105 was performed in horses, where blood and urine samples were collected over a time period of 96 h. The in vivo samples were compared with five in vitro incubation models encompassing Cunninghamella elegans, microsomes and S9 fractions of both human and equine origin. The analyses were performed using ultra-high performance liquid chromatography coupled to high resolution Q ExactiveTM OrbitrapTM mass spectrometry (UHPLC-HRMS). A total of 21 metabolites were tentatively identified from the in vivo experiments, of which several novel glucuronides were detected in plasma and urine. In hydrolyzed urine, hydroxylated metabolites dominated. The in vitro models yielded several biotransformation products, including a number of monohydroxylated metabolites matching the in vivo results. The suggested analytical target for equine doping control in plasma is a dihydroxylated metabolite with a net loss of two hydrogens. In urine, the suggested targets are two monohydroxylated metabolites after hydrolysis with β-glucuronidase, selected both due to prolongation of the detection time and the availability of reference material from the in vitro models.
Keywords: ACP-105; Cunninghamella elegans; SARM; Selective Androgen Receptor Modulator; doping; horse; mass spectrometry; metabolites; microsomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Papanicolaou D.A., Ather S.N., Zhu H., Zhou Y., Lutkiewicz J., Scott B.B., Chandler J. A phase IIA randomized, placebo-controlled clinical trial to study the efficacy and safety of the selective androgen receptor modulator (SARM), MK-0773 in female participants with sarcopenia. J. Nutr. Health Aging. 2013;17:533–543. doi: 10.1007/s12603-013-0335-x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

