Plant Seed Mucilage as a Glue: Adhesive Properties of Hydrated and Dried-in-Contact Seed Mucilage of Five Plant Species
- PMID: 33535533
- PMCID: PMC7867067
- DOI: 10.3390/ijms22031443
Plant Seed Mucilage as a Glue: Adhesive Properties of Hydrated and Dried-in-Contact Seed Mucilage of Five Plant Species
Abstract
Seed and fruit mucilage is composed of three types of polysaccharides-pectins, cellulose, and hemicelluloses-and demonstrates adhesive properties after hydration. One of the important functions of the mucilage is to enable seeds to attach to diverse natural surfaces. Due to its adhesive properties, which increase during dehydration, the diaspore can be anchored to the substrate (soil) or attached to an animal's body and dispersed over varied distances. After complete desiccation, the mucilage envelope forms a thin transparent layer around the diaspore creating a strong bond to the substrate. In the present study, we examined the mucilaginous seeds of six different plant taxa (from genera Linum, Lepidium, Ocimum, Salvia and Plantago) and addressed two main questions: (1) How strong is the adhesive bond of the dried mucilage envelope? and (2) What are the differences in adhesion between different mucilage types? Generally, the dried mucilage envelope revealed strong adhesive properties. Some differences between mucilage types were observed, particularly in relation to adhesive force (Fad) whose maximal values varied from 0.58 to 6.22 N. The highest adhesion force was revealed in the cellulose mucilage of Ocimum basilicum. However, mucilage lacking cellulose fibrils, such as that of Plantago ovata, also demonstrated high values of adhesion force with a maximum close to 5.74 N. The adhesion strength, calculated as force per unit contact area (Fad/A0), was comparable between studied taxa. Obtained results demonstrated (1) that the strength of mucilage adhesive bonds strongly surpasses the requirements necessary for epizoochory and (2) that seed mucilage has a high potential as a nontoxic, natural substance that can be used in water-based glues.
Keywords: Linum; Ocimum; Plantago; cell wall; diaspores; seed mucilage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
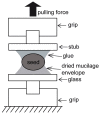
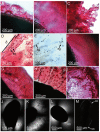


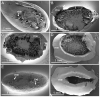

References
-
- Donaldson L. Cellulose microfibril aggregates and their size variation with cell wall type. Wood. Sci. Technol. 2007;41:443–460. doi: 10.1007/s00226-006-0121-6. - DOI
-
- Jarvis M.C. Plant cell walls: Supramolecular assembly, signalling and stress. Struct. Chem. 2009;20:245–253. doi: 10.1007/s11224-009-9427-y. - DOI
-
- Kreitschitz A., Gorb S.N. How does the cell wall ‘stick’ in the mucilage? A detailed microstructural analysis of the seed coat mucilaginous cell wall. Flora. 2017;229:9–22. doi: 10.1016/j.flora.2017.02.010. - DOI
-
- Grubert M. Studies on the distribution of myxospermy among seeds and fruits of Angiospermae and its ecological importance. Acta Biol. Venez. 1974;8:315–551.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

