Phosphatidic Acid Stimulates Myoblast Proliferation through Interaction with LPA1 and LPA2 Receptors
- PMID: 33535610
- PMCID: PMC7867176
- DOI: 10.3390/ijms22031452
Phosphatidic Acid Stimulates Myoblast Proliferation through Interaction with LPA1 and LPA2 Receptors
Abstract
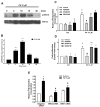
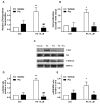
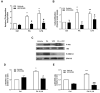
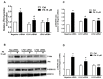
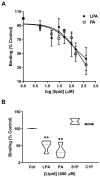
Phosphatidic acid (PA) is a bioactive phospholipid capable of regulating key biological functions, including neutrophil respiratory burst, chemotaxis, or cell growth and differentiation. However, the mechanisms whereby PA exerts these actions are not completely understood. In this work, we show that PA stimulates myoblast proliferation, as determined by measuring the incorporation of [3H]thymidine into DNA and by staining the cells with crystal violet. PA induced the rapid phosphorylation of Akt and ERK1/2, and pretreatment of the cells with specific small interferin RNA (siRNA) to silence the genes encoding these kinases, or with selective pharmacologic inhibitors, blocked PA-stimulated myoblast proliferation. The mitogenic effects of PA were abolished by the preincubation of the myoblasts with pertussis toxin, a Gi protein inhibitor, suggesting the implication of Gi protein-coupled receptors in this action. Although some of the effects of PA have been associated with its possible conversion to lysoPA (LPA), treatment of the myoblasts with PA for up to 60 min did not produce any significant amount of LPA in these cells. Of interest, pharmacological blockade of the LPA receptors 1 and 2, or specific siRNA to silence the genes encoding these receptors, abolished PA-stimulated myoblast proliferation. Moreover, PA was able to compete with LPA for binding to LPA receptors, suggesting that PA can act as a ligand of LPA receptors. It can be concluded that PA stimulates myoblast proliferation through interaction with LPA1 and LPA2 receptors and the subsequent activation of the PI3K/Akt and MEK/ERK1-2 pathways, independently of LPA formation.
Keywords: lysophosphatidic acid; lysophosphatidic acid receptors; myoblast proliferation; phosphatidic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
LPA2 (EDG4) mediates Rho-dependent chemotaxis with lower efficacy than LPA1 (EDG2) in breast carcinoma cells.Am J Physiol Cell Physiol. 2007 May;292(5):C1927-33. doi: 10.1152/ajpcell.00400.2006. Am J Physiol Cell Physiol. 2007. PMID: 17496233
-
Phosphatidic Acid Stimulates Lung Cancer Cell Migration through Interaction with the LPA1 Receptor and Subsequent Activation of MAP Kinases and STAT3.Biomedicines. 2023 Jun 23;11(7):1804. doi: 10.3390/biomedicines11071804. Biomedicines. 2023. PMID: 37509443 Free PMC article.
-
Antidepressants activate the lysophosphatidic acid receptor LPA(1) to induce insulin-like growth factor-I receptor transactivation, stimulation of ERK1/2 signaling and cell proliferation in CHO-K1 fibroblasts.Biochem Pharmacol. 2015 Jun 15;95(4):311-23. doi: 10.1016/j.bcp.2015.04.002. Epub 2015 Apr 15. Biochem Pharmacol. 2015. PMID: 25888927
-
Two pathways for lysophosphatidic acid production.Biochim Biophys Acta. 2008 Sep;1781(9):513-8. doi: 10.1016/j.bbalip.2008.06.005. Epub 2008 Jun 24. Biochim Biophys Acta. 2008. PMID: 18621144 Review.
-
The role of lysophosphatidic acid in the physiology and pathology of the skin.Life Sci. 2019 Mar 1;220:194-200. doi: 10.1016/j.lfs.2018.12.040. Epub 2018 Dec 22. Life Sci. 2019. PMID: 30584899 Review.
Cited by
-
Evaluation of the Timing of Use of Phosphatidic Acid in the Diet on Growth Performance and Breast Meat Yield in Broilers.Animals (Basel). 2022 Dec 7;12(24):3446. doi: 10.3390/ani12243446. Animals (Basel). 2022. PMID: 36552366 Free PMC article.
-
Implication of Ceramide Kinase/C1P in Cancer Development and Progression.Cancers (Basel). 2022 Jan 4;14(1):227. doi: 10.3390/cancers14010227. Cancers (Basel). 2022. PMID: 35008391 Free PMC article. Review.
-
CHML regulates migration and invasion in hepatocellular carcinoma via transcriptional and metabolic reprogramming.Front Oncol. 2025 Aug 6;15:1575809. doi: 10.3389/fonc.2025.1575809. eCollection 2025. Front Oncol. 2025. PMID: 40842592 Free PMC article.
-
Single-cell transcriptome dynamics of the autotaxin-lysophosphatidic acid axis during muscle regeneration reveal proliferative effects in mesenchymal fibro-adipogenic progenitors.Front Cell Dev Biol. 2023 Feb 23;11:1017660. doi: 10.3389/fcell.2023.1017660. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36910157 Free PMC article.
-
Elevated Serum Levels of Acid Sphingomyelinase in Female Patients with Episodic and Chronic Migraine.Antioxidants (Basel). 2025 Jan 29;14(2):159. doi: 10.3390/antiox14020159. Antioxidants (Basel). 2025. PMID: 40002346 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

