Clinical trials in a COVID-19 pandemic: Shared infrastructure for continuous learning in a rapidly changing landscape
- PMID: 33535821
- PMCID: PMC8172421
- DOI: 10.1177/1740774520988298
Clinical trials in a COVID-19 pandemic: Shared infrastructure for continuous learning in a rapidly changing landscape
Abstract
Background: Clinical trials, conducted efficiently and with the utmost integrity, are a key component in identifying effective vaccines, therapies, and other interventions urgently needed to solve the COVID-19 crisis. Yet launching and implementing trials with the rigor necessary to produce convincing results is a complicated and time-consuming process. Balancing rigor and efficiency involves relying on designs that employ flexible features to respond to a fast-changing landscape, measuring valid endpoints that result in translational actions and disseminating findings in a timely manner. We describe the challenges involved in creating infrastructure with potential utility for shared learning.
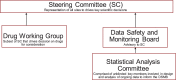
Methods: We have established a shared infrastructure that borrows strength across multiple trials. The infrastructure includes an endpoint registry to aid in selecting appropriate endpoints, a registry to facilitate establishing a Data & Safety Monitoring Board, common data collection instruments, a COVID-19 dedicated design and analysis team, and a pragmatic platform protocol, among other elements.
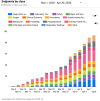
Results: The authors have relied on the shared infrastructure for six clinical trials for which they serve as the Data Coordinating Center and have a design and analysis team comprising 15 members who are dedicated to COVID-19. The authors established a pragmatic platform to simultaneously investigate multiple treatments for the outpatient with adaptive features to add or drop treatment arms.
Conclusion: The shared infrastructure provides appealing opportunities to evaluate disease in a more robust manner with fewer resources and is especially valued during a pandemic where efficiency in time and resources is crucial. The most important element of the shared infrastructure is the pragmatic platform. While it may be the most challenging of the elements to establish, it may provide the greatest benefit to both patients and researchers.
Keywords: COVID-19; Data & Safety Monitoring Board; Data Coordinating Center; Platform protocol; core protocol; efficient infrastructure; master protocol; pandemic.
Conflict of interest statement
Figures



References
-
- Dunn A. There are already 72 drugs in human trials for coronavirus in the US with hundreds more on the way, a top drug regulator warns we could run out of researchers to test them all. Business Insider, 2020, https://www.businessinsider.com/fda-woodcock-overwhelming-amount-of-coro... (2020, accessed 20 July 2020).
-
- Guideline IH. Guideline for good clinical practice. J Postgrad Med 2001; 47(3): 199–203. - PubMed
-
- World Health Organization. Handbook for good clinical research practice (GCP): guidance for implementation, https://www.who.int/medicines/areas/quality_safety/safety_efficacy/gcp1.pdf (2005, accessed 20 July 2020).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

