Construct validity of the Post-COVID-19 Functional Status Scale in adult subjects with COVID-19
- PMID: 33536042
- PMCID: PMC7856622
- DOI: 10.1186/s12955-021-01691-2
Construct validity of the Post-COVID-19 Functional Status Scale in adult subjects with COVID-19
Abstract
Background: An increasing number of subjects are recovering from COVID-19, raising the need for tools to adequately assess the course of the disease and its impact on functional status. We aimed to assess the construct validity of the Post-COVID-19 Functional Status (PCFS) Scale among adult subjects with confirmed and presumed COVID-19.
Methods: Adult subjects with confirmed and presumed COVID-19, who were members of an online panel and two Facebook groups for subjects with COVID-19 with persistent symptoms, completed an online survey after the onset of infection-related symptoms. The number and intensity of symptoms were evaluated with the Utrecht Symptom Diary, health-related quality of life (HrQoL) with the 5-level EQ-5D questionnaire, impairment in work and activities with the Work Productivity and Activity Impairment questionnaire and functional status with the PCFS Scale.
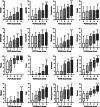
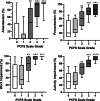
Results: 1939 subjects were included in the analyses (85% women, 95% non-hospitalized during infection) about 3 months after the onset of infection-related symptoms. Subjects classified as experiencing 'slight', 'moderate' and 'severe' functional limitations presented a gradual increase in the number/intensity of symptoms, reduction of HrQoL and impairment in work and usual activities. No differences were found regarding the number and intensity of symptoms, HrQoL and impairment in work and usual activities between subjects classified as experiencing 'negligible' and 'no' functional limitations. We found weak-to-strong statistical associations between functional status and all domains of HrQoL (r: 0.233-0.661). Notably, the strongest association found was with the 'usual activities' domain of the 5-level EQ-5D questionnaire.
Conclusion: We demonstrated the construct validity of the PCFS Scale in highly-symptomatic adult subjects with confirmed and presumed COVID-19, 3 months after the onset of symptoms.
Keywords: Functional status; Quality of life; SARS-CoV-2; Symptoms.
Conflict of interest statement
FMEF reports grants and personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Chiesi, personal fees from GlaxoSmithKline, grants and personal fees from Novartis, and personal fees from TEVA, outside the submitted work. DJAJ has received speaker fees from AstraZeneca, Boehringer Ingelheim and Novartis, outside the submitted work. FAK has received research grants from Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, MSD, Daiichi-Sankyo, Actelion, the Dutch thrombosis association, the Netherlands Organisation for Health Research and Development and the Dutch Heart foundation, all outside this work. MAS reports grants from Netherlands Lung Foundation, grants and personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, and grants from Stichting Astma Bestrijding, all outside the submitted work. All other authors declare that they have no conflicts of interest.
Figures

References
-
- WHO. Weekly operational update on COVID-19. https://www.who.int/publications/m/item/weekly-epidemiological-update---.... Accessed 17 Jan 2021.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

