Cannabinoid treatment for autism: a proof-of-concept randomized trial
- PMID: 33536055
- PMCID: PMC7860205
- DOI: 10.1186/s13229-021-00420-2
Cannabinoid treatment for autism: a proof-of-concept randomized trial
Abstract
Background: Endocannabinoid dysfunction in animal models of autism spectrum disorder (ASD) and accumulating, albeit anecdotal, evidence for efficacy in humans motivated this placebo-controlled double-blind comparison of two oral cannabinoid solutions in 150 participants (age 5-21 years) with ASD.
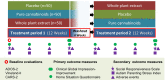
Methods: We tested (1) BOL-DP-O-01-W, a whole-plant cannabis extract containing cannabidiol and Δ9-tetrahydrocannabinol at a 20:1 ratio and (2) BOL-DP-O-01, purified cannabidiol and Δ9-tetrahydrocannabinol at the same ratio. Participants (N = 150) received either placebo or cannabinoids for 12-weeks (testing efficacy) followed by a 4-week washout and predetermined cross-over for another 12 weeks to further assess tolerability. Registered primary efficacy outcome measures were improvement in behavioral problems (differences between whole-plant extract and placebo) on the Home Situation Questionnaire-ASD (HSQ-ASD) and the Clinical Global Impression-Improvement scale with disruptive behavior anchor points (CGI-I). Secondary measures were Social Responsiveness Scale (SRS-2) and Autism Parenting Stress Index (APSI).
Results: Changes in Total Scores of HSQ-ASD (primary-outcome) and APSI (secondary-outcome) did not differ among groups. Disruptive behavior on the CGI-I (co-primary outcome) was either much or very much improved in 49% on whole-plant extract (n = 45) versus 21% on placebo (n = 47; p = 0.005). Median SRS Total Score (secondary-outcome) improved by 14.9 on whole-plant extract (n = 34) versus 3.6 points after placebo (n = 36); p = 0.009). There were no treatment-related serious adverse events. Common adverse events included somnolence and decreased appetite, reported for 28% and 25% on whole-plant extract, respectively (n = 95); 23% and 21% on pure-cannabinoids (n = 93), and 8% and 15% on placebo (n = 94). Limitations Lack of pharmacokinetic data and a wide range of ages and functional levels among participants warrant caution when interpreting the results.
Conclusions: This interventional study provides evidence that BOL-DP-O-01-W and BOL-DP-O-01, administrated for 3 months, are well tolerated. Evidence for efficacy of these interventions are mixed and insufficient. Further testing of cannabinoids in ASD is recommended. Trial registration ClinicalTrials.gov: NCT02956226. Registered 06 November 2016, https://clinicaltrials.gov/ct2/show/NCT02956226.
Keywords: Autism spectrum disorder; Behavior; Cannabidiol; Cannabinoids; Child psychiatry; Clinical trials randomized controlled; Developmental disorders; Entourage effect; Neuropsychology; Tetrahydrocannabinol.
Conflict of interest statement
Adi Aran and F. Xavier Castellanos report receiving personal fees and stock options for advisory roles at BOL Pharma. The remaining authors have no conflicts of interest to disclose.
Figures



References
-
- Szkudlarek HJ, Desai SJ, Renard J, Pereira B, Norris C, Jobson CEL, et al. Delta-9-Tetrahydrocannabinol and Cannabidiol produce dissociable effects on prefrontal cortical executive function and regulation of affective behaviors. Neuropsychopharmacology. 2019;44(4):817–825. doi: 10.1038/s41386-018-0282-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

