Nonsegmented Negative-Sense RNA Viruses Utilize N6-Methyladenosine (m6A) as a Common Strategy To Evade Host Innate Immunity
- PMID: 33536170
- PMCID: PMC8104112
- DOI: 10.1128/JVI.01939-20
Nonsegmented Negative-Sense RNA Viruses Utilize N6-Methyladenosine (m6A) as a Common Strategy To Evade Host Innate Immunity
Abstract
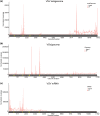
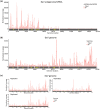
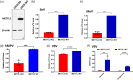
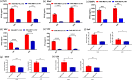
N6-Methyladenosine (m6A) is the most abundant internal RNA modification catalyzed by host RNA methyltransferases. As obligate intracellular parasites, many viruses acquire m6A methylation in their RNAs. However, the biological functions of viral m6A methylation are poorly understood. Here, we found that viral m6A methylation serves as a molecular marker for host innate immunity to discriminate self from nonself RNA and that this novel biological function of viral m6A methylation is universally conserved in several families in nonsegmented negative-sense (NNS) RNA viruses. Using m6A methyltransferase (METTL3) knockout cells, we produced m6A-deficient virion RNAs from the representative members of the families Pneumoviridae, Paramyxoviridae, and Rhabdoviridae and found that these m6A-deficient viral RNAs triggered significantly higher levels of type I interferon compared to the m6A-sufficient viral RNAs, in a RIG-I-dependent manner. Reconstitution of the RIG-I pathway revealed that m6A-deficient virion RNA induced higher expression of RIG-I, bound to RIG-I more efficiently, enhanced RIG-I ubiquitination, and facilitated RIG-I conformational rearrangement and oligomerization. Furthermore, the m6A binding protein YTHDF2 is essential for suppression of the type I interferon signaling pathway, including by virion RNA. Collectively, our results suggest that several families in NNS RNA viruses acquire m6A in viral RNA as a common strategy to evade host innate immunity.IMPORTANCE The nonsegmented negative-sense (NNS) RNA viruses share many common replication and gene expression strategies. There are no vaccines or antiviral drugs for many of these viruses. We found that representative members of the families Pneumoviridae, Paramyxoviridae, and Rhabdoviridae among the NNS RNA viruses acquire m6A methylation in their genome and antigenome as a means to escape recognition by host innate immunity via a RIG-I-dependent signaling pathway. Viral RNA lacking m6A methylation induces a significantly higher type I interferon response than m6A-sufficient viral RNA. In addition to uncovering m6A methylation as a common mechanism for many NNS RNA viruses to evade host innate immunity, this study discovered a novel strategy to enhance type I interferon responses, which may have important applications in vaccine development, as robust innate immunity will likely promote the subsequent adaptive immunity.
Keywords: N6-methyladenosine; innate immunity; negative-strand RNA virus.
Copyright © 2021 American Society for Microbiology.
Figures


Similar articles
-
The Role of Chemical Modifications in the Genome of Negative-Sense RNA Viruses on the Innate Immune Response.Viruses. 2025 May 30;17(6):795. doi: 10.3390/v17060795. Viruses. 2025. PMID: 40573386 Free PMC article. Review.
-
N6-methyladenosine modification enables viral RNA to escape recognition by RNA sensor RIG-I.Nat Microbiol. 2020 Apr;5(4):584-598. doi: 10.1038/s41564-019-0653-9. Epub 2020 Feb 3. Nat Microbiol. 2020. PMID: 32015498 Free PMC article.
-
N6-Methyladenosine modification of hepatitis B and C viral RNAs attenuates host innate immunity via RIG-I signaling.J Biol Chem. 2020 Sep 11;295(37):13123-13133. doi: 10.1074/jbc.RA120.014260. Epub 2020 Jul 27. J Biol Chem. 2020. PMID: 32719095 Free PMC article.
-
N6-methyladenosine RNA modification suppresses antiviral innate sensing pathways via reshaping double-stranded RNA.Nat Commun. 2021 Mar 11;12(1):1582. doi: 10.1038/s41467-021-21904-y. Nat Commun. 2021. PMID: 33707441 Free PMC article.
-
Regulation of Viral Infection by the RNA Modification N6-Methyladenosine.Annu Rev Virol. 2019 Sep 29;6(1):235-253. doi: 10.1146/annurev-virology-092818-015559. Epub 2019 Jul 5. Annu Rev Virol. 2019. PMID: 31283446 Free PMC article. Review.
Cited by
-
Post-Transcriptional Regulation of Viral RNA through Epitranscriptional Modification.Cells. 2021 May 7;10(5):1129. doi: 10.3390/cells10051129. Cells. 2021. PMID: 34066974 Free PMC article. Review.
-
m6A Reader YTHDC1 Impairs Respiratory Syncytial Virus Infection by Downregulating Membrane CX3CR1 Expression.Viruses. 2024 May 14;16(5):778. doi: 10.3390/v16050778. Viruses. 2024. PMID: 38793659 Free PMC article.
-
N6-methyladenosine modification positively regulate Japanese encephalitis virus replication.Virol J. 2024 Jan 19;21(1):23. doi: 10.1186/s12985-023-02275-w. Virol J. 2024. PMID: 38243270 Free PMC article.
-
The Battle for Survival: The Role of RNA Non-Canonical Tails in the Virus-Host Interaction.Metabolites. 2023 Sep 13;13(9):1009. doi: 10.3390/metabo13091009. Metabolites. 2023. PMID: 37755289 Free PMC article. Review.
-
m6A modification-mediated BATF2 suppresses metastasis and angiogenesis of tongue squamous cell carcinoma through inhibiting VEGFA.Cell Cycle. 2023 Jan;22(1):100-116. doi: 10.1080/15384101.2022.2109897. Epub 2022 Aug 10. Cell Cycle. 2023. PMID: 35949109 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

