Prominent Neutralizing Antibody Response Targeting the Ebolavirus Glycoprotein Subunit Interface Elicited by Immunization
- PMID: 33536172
- PMCID: PMC8103683
- DOI: 10.1128/JVI.01907-20
Prominent Neutralizing Antibody Response Targeting the Ebolavirus Glycoprotein Subunit Interface Elicited by Immunization
Abstract
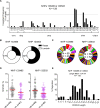
The severe death toll caused by the recent outbreak of Ebola virus disease reinforces the importance of developing ebolavirus prevention and treatment strategies. Here, we have explored the immunogenicity of a novel immunization regimen priming with vesicular stomatitis virus particles bearing Sudan Ebola virus (SUDV) glycoprotein (GP) that consists of GP1 & GP2 subunits and boosting with soluble SUDV GP in macaques, which developed robust neutralizing antibody (nAb) responses following immunizations. Moreover, EB46, a protective nAb isolated from one of the immune macaques, is found to target the GP1/GP2 interface, with GP-binding mode and neutralization mechanism similar to a number of ebolavirus nAbs from human and mouse, indicating that the ebolavirus GP1/GP2 interface is a common immunological target in different species. Importantly, selected immune macaque polyclonal sera showed nAb specificity similar to EB46 at substantial titers, suggesting that the GP1/GP2 interface region is a viable target for ebolavirus vaccine.Importance: The elicitation of sustained neutralizing antibody (nAb) responses against diverse ebolavirus strains remains as a high priority for the vaccine field. The most clinically advanced rVSV-ZEBOV vaccine could elicit moderate nAb responses against only one ebolavirus strain, EBOV, among the five ebolavirus strains, which last less than 6 months. Boost immunization strategies are desirable to effectively recall the rVSV vector-primed nAb responses to prevent infections in prospective epidemics, while an in-depth understanding of the specificity of immunization-elicited nAb responses is essential for improving vaccine performance. Here, using non-human primate animal model, we demonstrated that booster immunization with a stabilized trimeric soluble form of recombinant glycoprotein derived from the ebolavirus Sudan strain following the priming rVSV vector immunization led to robust nAb responses that substantially map to the subunit interface of ebolavirus glycoprotein, a common B cell repertoire target of multiple species including primates and rodents.
Copyright © 2021 American Society for Microbiology.
Figures




Similar articles
-
Antibody-Mediated Protective Mechanisms Induced by a Trivalent Parainfluenza Virus-Vectored Ebolavirus Vaccine.J Virol. 2019 Feb 5;93(4):e01845-18. doi: 10.1128/JVI.01845-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30518655 Free PMC article.
-
Distinct Immunogenicity and Efficacy of Poxvirus-Based Vaccine Candidates against Ebola Virus Expressing GP and VP40 Proteins.J Virol. 2018 May 14;92(11):e00363-18. doi: 10.1128/JVI.00363-18. Print 2018 Jun 1. J Virol. 2018. PMID: 29514907 Free PMC article.
-
A Bivalent, Spherical Virus-Like Particle Vaccine Enhances Breadth of Immune Responses against Pathogenic Ebola Viruses in Rhesus Macaques.J Virol. 2020 Apr 16;94(9):e01884-19. doi: 10.1128/JVI.01884-19. Print 2020 Apr 16. J Virol. 2020. PMID: 32075939 Free PMC article.
-
Correlates of vaccine-induced protective immunity against Ebola virus disease.Semin Immunol. 2018 Oct;39:65-72. doi: 10.1016/j.smim.2018.07.003. Epub 2018 Jul 21. Semin Immunol. 2018. PMID: 30041831 Review.
-
Durability of single-dose rVSV-ZEBOV vaccine responses: what do we know?Expert Rev Vaccines. 2018 Dec;17(12):1105-1110. doi: 10.1080/14760584.2018.1546582. Epub 2018 Nov 15. Expert Rev Vaccines. 2018. PMID: 30422031 Review.
Cited by
-
One dose of COVID-19 nanoparticle vaccine REVC-128 protects against SARS-CoV-2 challenge at two weeks post-immunization.Emerg Microbes Infect. 2021 Dec;10(1):2016-2029. doi: 10.1080/22221751.2021.1994354. Emerg Microbes Infect. 2021. PMID: 34651563 Free PMC article.
-
Design of universal Ebola virus vaccine candidates via immunofocusing.bioRxiv [Preprint]. 2023 Oct 17:2023.10.14.562364. doi: 10.1101/2023.10.14.562364. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2024 Feb 13;121(7):e2316960121. doi: 10.1073/pnas.2316960121. PMID: 37904982 Free PMC article. Updated. Preprint.
-
Single-vial filovirus glycoprotein vaccines: Biophysical characteristics and immunogenicity after co-lyophilization with adjuvant.Vaccine. 2021 Sep 15;39(39):5650-5657. doi: 10.1016/j.vaccine.2021.08.003. Epub 2021 Aug 13. Vaccine. 2021. PMID: 34400019 Free PMC article.
-
Design of universal Ebola virus vaccine candidates via immunofocusing.Proc Natl Acad Sci U S A. 2024 Feb 13;121(7):e2316960121. doi: 10.1073/pnas.2316960121. Epub 2024 Feb 6. Proc Natl Acad Sci U S A. 2024. PMID: 38319964 Free PMC article.
-
Production and Purification of Filovirus Glycoproteins.Methods Mol Biol. 2024;2762:17-25. doi: 10.1007/978-1-0716-3666-4_2. Methods Mol Biol. 2024. PMID: 38315357
References
-
- Albarino CG, Shoemaker T, Khristova ML, Wamala JF, Muyembe JJ, Balinandi S, Tumusiime A, Campbell S, Cannon D, Gibbons A, Bergeron E, Bird B, Dodd K, Spiropoulou C, Erickson BR, Guerrero L, Knust B, Nichol ST, Rollin PE, Stroher U. 2013. Genomic analysis of filoviruses associated with four viral hemorrhagic fever outbreaks in Uganda and the Democratic Republic of the Congo in 2012. Virology 442:97–100. doi:10.1016/j.virol.2013.04.014. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

