ONC201 Shows Potent Anticancer Activity Against Medullary Thyroid Cancer via Transcriptional Inhibition of RET, VEGFR2, and IGFBP2
- PMID: 33536187
- PMCID: PMC9484041
- DOI: 10.1158/1535-7163.MCT-20-0386
ONC201 Shows Potent Anticancer Activity Against Medullary Thyroid Cancer via Transcriptional Inhibition of RET, VEGFR2, and IGFBP2
Abstract
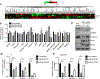
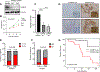
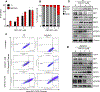
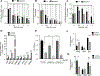
Gain-of-function point mutations in the receptor tyrosine kinase RET, a driver oncogene in medullary thyroid carcinoma (MTC), prevent apoptosis through inhibition of ATF4, a critical transcriptional regulator of endoplasmic reticulum stress. However, the critical regulatory mechanisms driving RET-dependent oncogenesis remain elusive, and there is a clinical need to identify a transcriptional RET inhibitor. Here, we found that RET depletion decreased IGFBP2 and VEGFR2 mRNA and protein expression in MTC cells. IGFBP2 knockdown decreased cell survival and migration of MTC cells. In patients, IGFBP2 expression increased in metastatic MTC, and high IGFBP2 associated with poor overall survival. VEGFR2 protein levels were positively associated with RET expression in primary tumors, and VEGF-mediated increased cell viability was RET dependent. The small-molecule ONC201 treatment of MTC cells caused apoptotic cell death, decreased transcription of RET, VEGFR2, IGFBP2, increased mRNA levels of ATF4, and ATF4 target genes including DDIT3, BBC3, DUSP8, MKNK2, KLF9, LZTFL1, and SESN2 Moreover, IGFBP2 depletion increased ONC201-induced cell death. ONC201 inhibited tumor growth at a well-tolerated dose of 120 mg/kg/week administered by oral gavage and decreased MTC xenograft cell proliferation and angiogenesis. The protein levels of RET, IGFBP2, and VEGFR2 were decreased in ONC201-treated xenografts. Our study uncovered a novel ONC201 mechanism of action through regulation of RET and its targets, VEGFR2 and IGFBP2; this mechanism could be translated into the clinic and represent a promising strategy for the treatment of all patients with MTC, including those with TKI-refractory disease and other cancer with RET abnormalities.
©2021 American Association for Cancer Research.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

