On-demand biomanufacturing of protective conjugate vaccines
- PMID: 33536221
- PMCID: PMC7857678
- DOI: 10.1126/sciadv.abe9444
On-demand biomanufacturing of protective conjugate vaccines
Abstract
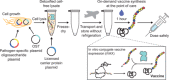
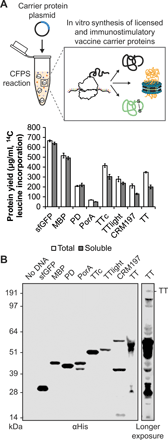
Conjugate vaccines are among the most effective methods for preventing bacterial infections. However, existing manufacturing approaches limit access to conjugate vaccines due to centralized production and cold chain distribution requirements. To address these limitations, we developed a modular technology for in vitro conjugate vaccine expression (iVAX) in portable, freeze-dried lysates from detoxified, nonpathogenic Escherichia coli. Upon rehydration, iVAX reactions synthesize clinically relevant doses of conjugate vaccines against diverse bacterial pathogens in 1 hour. We show that iVAX-synthesized vaccines against Francisella tularensis subsp. tularensis (type A) strain Schu S4 protected mice from lethal intranasal F. tularensis challenge. The iVAX platform promises to accelerate development of new conjugate vaccines with increased access through refrigeration-independent distribution and portable production.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- Review on Antimicrobial Resistance, Antimicrobial resistance: Tackling a crisis for the health and wealth of nations (2014); https://wellcomecollection.org/works/rdpck35v.
-
- Weintraub A., Immunology of bacterial polysaccharide antigens. Carbohydr. Res. 338, 2539–2547 (2003). - PubMed
-
- Trotter C. L., Vernon J. M., Ramsay M. E., Whitney C. G., Mulholland E. K., Goldblatt D., Hombach J., Kieny M.-P.; SAGE subgroup , Optimising the use of conjugate vaccines to prevent disease caused by Haemophilus influenzae type b, Neisseria meningitidis and Streptococcus pneumoniae. Vaccine 26, 4434–4445 (2008). - PubMed
-
- Jin C., Gibani M. M., Moore M., Juel H. B., Jones E., Meiring J., Harris V., Gardner J., Nebykova A., Kerridge S. A., Hill J., Thomaides-Brears H., Blohmke C. J., Yu L.-M., Angus B., Pollard A. J., Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi: A randomised controlled, phase 2b trial. Lancet 390, 2472–2480 (2017). - PMC - PubMed
-
- Novak R. T., Kambou J. L., Diomandé F. V., Tarbangdo T. F., Ouédraogo-Traoré R., Sangaré L., Lingani C., Martin S. W., Hatcher C., Mayer L. W., Laforce F. M., Avokey F., Djingarey M. H., Messonnier N. E., Tiendrébéogo S. R., Clark T. A., Serogroup A meningococcal conjugate vaccination in Burkina Faso: Analysis of national surveillance data. Lancet Infect. Dis. 12, 757–764 (2012). - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

