Eosinophils attenuate hepatic ischemia-reperfusion injury in mice through ST2-dependent IL-13 production
- PMID: 33536281
- PMCID: PMC8167890
- DOI: 10.1126/scitranslmed.abb6576
Eosinophils attenuate hepatic ischemia-reperfusion injury in mice through ST2-dependent IL-13 production
Abstract
Eosinophils are a myeloid cell subpopulation that mediates type 2 T helper cell immune responses. Unexpectedly, we identified a rapid accumulation of eosinophils in 22 human liver grafts after hepatic transplantation. In contrast, no eosinophils were detectable in healthy liver tissues before transplantation. Studies with two genetic mouse models of eosinophil deficiency and a mouse model of antibody-mediated eosinophil depletion revealed exacerbated liver injury after hepatic ischemia and reperfusion. Adoptive transfer of bone marrow-derived eosinophils normalized liver injury of eosinophil-deficient mice and reduced hepatic ischemia and reperfusion injury in wild-type mice. Mechanistic studies combining genetic and adoptive transfer approaches identified a critical role of suppression of tumorigenicity (ST2)-dependent production of interleukin-13 by eosinophils in the hepatoprotection against ischemia-reperfusion-induced injury. Together, these data provide insight into a mechanism of eosinophil-mediated liver protection that could serve as a therapeutic target to improve outcomes of patients undergoing liver transplantation.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


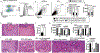



References
-
- Hilmi I, Horton CN, Planinsic RM, Sakai T, Nicolau-Raducu R, Damian D, Gligor S, Marcos A, The impact of postreperfusion syndrome on short-term patient and liver allograft outcome in patients undergoing orthotopic liver transplantation. Liver. Transpl 14, 504–508 (2008). - PubMed
-
- Watt KDS, Lyden ER, Gulizia JM, McCashland TM, Recurrent hepatitis C posttransplant: Early preservation injury may predict poor outcome. Liver Transpl. 12, 134–139 (2006). - PubMed
-
- Busuttil RW, Tanaka K, The utility of marginal donors in liver transplantation. Liver Transpl. 9, 651–663 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL114457/HL/NHLBI NIH HHS/United States
- R01 HL109233/HL/NHLBI NIH HHS/United States
- R01 HL124165/HL/NHLBI NIH HHS/United States
- R21 AA024636/AA/NIAAA NIH HHS/United States
- R01 ES019311/ES/NIEHS NIH HHS/United States
- P01 AI116501/AI/NIAID NIH HHS/United States
- R21 AI132840/AI/NIAID NIH HHS/United States
- R01 DK097075/DK/NIDDK NIH HHS/United States
- R01 DK122708/DK/NIDDK NIH HHS/United States
- R01 DK109574/DK/NIDDK NIH HHS/United States
- R01 HL119837/HL/NHLBI NIH HHS/United States
- R01 DK121330/DK/NIDDK NIH HHS/United States
- R01 AI145108/AI/NIAID NIH HHS/United States
- R01 HL133900/HL/NHLBI NIH HHS/United States
- R01 DK122796/DK/NIDDK NIH HHS/United States
- U01 AA021723/AA/NIAAA NIH HHS/United States
- R01 HL065228/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

