Pharmacokinetics and Efficacy of a Potential Smallpox Therapeutic, Brincidofovir, in a Lethal Monkeypox Virus Animal Model
- PMID: 33536322
- PMCID: PMC7860987
- DOI: 10.1128/mSphere.00927-20
Pharmacokinetics and Efficacy of a Potential Smallpox Therapeutic, Brincidofovir, in a Lethal Monkeypox Virus Animal Model
Erratum in
-
Correction for Hutson et al., "Pharmacokinetics and Efficacy of a Potential Smallpox Therapeutic, Brincidofovir, in a Lethal Monkeypox Virus Animal Model".mSphere. 2021 Feb 17;6(1):e00126-21. doi: 10.1128/mSphere.00126-21. mSphere. 2021. PMID: 33597175 Free PMC article. No abstract available.
Abstract
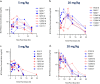
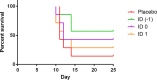
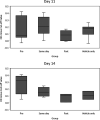
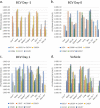
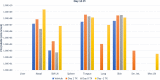
Smallpox, caused by Variola virus (VARV), was eradicated in 1980; however, VARV bioterrorist threats still exist, necessitating readily available therapeutics. Current preparedness activities recognize the importance of oral antivirals and recommend therapeutics with different mechanisms of action. Monkeypox virus (MPXV) is closely related to VARV, causing a highly similar clinical human disease, and can be used as a surrogate for smallpox antiviral testing. The prairie dog MPXV model has been characterized and used to study the efficacy of antipoxvirus therapeutics, including recently approved TPOXX (tecovirimat). Brincidofovir (BCV; CMX001) has shown antiviral activity against double-stranded DNA viruses, including poxviruses. To determine the exposure of BCV following oral administration to prairie dogs, a pharmacokinetics (PK) study was performed. Analysis of BCV plasma concentrations indicated variability, conceivably due to the outbred nature of the animals. To determine BCV efficacy in the MPXV prairie dog model, groups of animals were intranasally challenged with 9 × 105 plaque-forming units (PFU; 90% lethal dose [LD90]) of MPXV on inoculation day 0 (ID0). Animals were divided into groups based on the first day of BCV treatment relative to inoculation day (ID-1, ID0, or ID1). A trend in efficacy was noted dependent upon treatment initiation (57% on ID-1, 43% on ID0, and 29% on ID1) but was lower than demonstrated in other animal models. Analysis of the PK data indicated that BCV plasma exposure (maximum concentration [Cmax]) and the time of the last quantifiable concentration (AUClast) were lower than in other animal models administered the same doses, indicating that suboptimal BCV exposure may explain the lower protective effect on survival.IMPORTANCE Preparedness activities against highly transmissible viruses with high mortality rates have been highlighted during the ongoing coronavirus disease 2019 (COVID-19) pandemic. Smallpox, caused by variola virus (VARV) infection, is highly transmissible, with an estimated 30% mortality. Through an intensive vaccination campaign, smallpox was declared eradicated in 1980, and routine smallpox vaccination of individuals ceased. Today's current population has little/no immunity against VARV. If smallpox were to reemerge, the worldwide results would be devastating. Recent FDA approval of one smallpox antiviral (tecovirimat) was a successful step in biothreat preparedness; however, orthopoxviruses can become resistant to treatment, suggesting the need for multiple therapeutics. Our paper details the efficacy of the investigational smallpox drug brincidofovir in a monkeypox virus (MPXV) animal model. Since brincidofovir has not been tested in vivo against smallpox, studies with the related virus MPXV are critical in understanding whether it would be protective in the event of a smallpox outbreak.
Keywords: animal models; experimental therapeutics; monkeypox; smallpox; virology.
Figures
References
-
- Ogoina D, Izibewule JH, Ogunleye A, Ederiane E, Anebonam U, Neni A, Oyeyemi A, Etebu EN, Ihekweazu C. 2019. The 2017 human monkeypox outbreak in Nigeria—report of outbreak experience and response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLoS One 14:e0214229. doi:10.1371/journal.pone.0214229. - DOI - PMC - PubMed
-
- Reynolds MG, Wauquier N, Li Y, Satheshkumar PS, Kanneh LD, Monroe B, Maikere J, Saffa G, Gonzalez JP, Fair J, Carroll DS, Jambai A, Dafae F, Khan SH, Moses LM. 2019. Human monkeypox in Sierra Leone after 44-year absence of reported cases. Emerg Infect Dis 25:1023–1025. doi:10.3201/eid2505.180832. - DOI - PMC - PubMed
-
- Doshi RH, Guagliardo SAJ, Doty JB, Babeaux AD, Matheny A, Burgado J, Townsend MB, Morgan CN, Satheshkumar PS, Ndakala N, Kanjingankolo T, Kitembo L, Malekani J, Kalemba L, Pukuta E, N'Kaya T, Kangoula F, Moses C, McCollum AM, Reynolds MG, Mombouli JV, Nakazawa Y, Petersen BW. 2019. Epidemiologic and ecologic investigations of monkeypox, Likouala Department, Republic of the Congo, 2017. Emerg Infect Dis 25:281–289. doi:10.3201/eid2502.181222. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
