Effects of Seasonal Anoxia on the Microbial Community Structure in Demosponges in a Marine Lake in Lough Hyne, Ireland
- PMID: 33536324
- PMCID: PMC7860989
- DOI: 10.1128/mSphere.00991-20
Effects of Seasonal Anoxia on the Microbial Community Structure in Demosponges in a Marine Lake in Lough Hyne, Ireland
Abstract
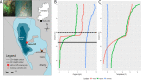
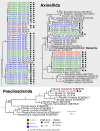
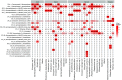
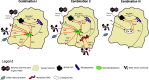
Climate change is expanding marine oxygen minimum zones (OMZs), while anthropogenic nutrient input depletes oxygen concentrations locally. The effects of deoxygenation on animals are generally detrimental; however, some sponges (Porifera) exhibit hypoxic and anoxic tolerance through currently unknown mechanisms. Sponges harbor highly specific microbiomes, which can include microbes with anaerobic capabilities. Sponge-microbe symbioses must also have persisted through multiple anoxic/hypoxic periods throughout Earth's history. Since sponges lack key components of the hypoxia-inducible factor (HIF) pathway responsible for hypoxic responses in other animals, it was hypothesized that sponge tolerance to deoxygenation may be facilitated by its microbiome. To test this hypothesis, we determined the microbial composition of sponge species tolerating seasonal anoxia and hypoxia in situ in a semienclosed marine lake, using 16S rRNA amplicon sequencing. We discovered a high degree of cryptic diversity among sponge species tolerating seasonal deoxygenation, including at least nine encrusting species of the orders Axinellida and Poecilosclerida. Despite significant changes in microbial community structure in the water, sponge microbiomes were species specific and remarkably stable under varied oxygen conditions, which was further explored for Eurypon spp. 2 and Hymeraphia stellifera However, some symbiont sharing occurred under anoxia. At least three symbiont combinations, all including large populations of Thaumarchaeota, corresponded with deoxygenation tolerance, and some combinations were shared between some distantly related hosts. We propose hypothetical host-symbiont interactions following deoxygenation that could confer deoxygenation tolerance.IMPORTANCE The oceans have an uncertain future due to anthropogenic stressors and an uncertain past that is becoming clearer with advances in biogeochemistry. Both past and future oceans were, or will be, deoxygenated in comparison to present conditions. Studying how sponges and their associated microbes tolerate deoxygenation provides insights into future marine ecosystems. Moreover, sponges form the earliest branch of the animal evolutionary tree, and they likely resemble some of the first animals. We determined the effects of variable environmental oxygen concentrations on the microbial communities of several demosponge species during seasonal anoxia in the field. Our results indicate that anoxic tolerance in some sponges may depend on their symbionts, but anoxic tolerance was not universal in sponges. Therefore, some sponge species could likely outcompete benthic organisms like corals in future, reduced-oxygen ecosystems. Our results support the molecular evidence that sponges and other animals have a Neoproterozoic origin and that animal evolution was not limited by low-oxygen conditions.
Keywords: Demospongiae; Porifera; Thaumarchaeota; anoxia; deoxygenation; host-microbe interactions; microbiome.
Copyright © 2021 Schuster et al.
Figures




Similar articles
-
Dynamic microbiome diversity shaping the adaptation of sponge holobionts in coastal waters.Microbiol Spectr. 2024 Nov 5;12(11):e0144824. doi: 10.1128/spectrum.01448-24. Epub 2024 Oct 14. Microbiol Spectr. 2024. PMID: 39400157 Free PMC article.
-
Stable symbionts across the HMA-LMA dichotomy: low seasonal and interannual variation in sponge-associated bacteria from taxonomically diverse hosts.FEMS Microbiol Ecol. 2015 Oct;91(10):fiv115. doi: 10.1093/femsec/fiv115. Epub 2015 Sep 23. FEMS Microbiol Ecol. 2015. PMID: 26405300
-
Diversity, structure and convergent evolution of the global sponge microbiome.Nat Commun. 2016 Jun 16;7:11870. doi: 10.1038/ncomms11870. Nat Commun. 2016. PMID: 27306690 Free PMC article.
-
The sponge holobiont in a changing ocean: from microbes to ecosystems.Microbiome. 2018 Mar 9;6(1):46. doi: 10.1186/s40168-018-0428-1. Microbiome. 2018. PMID: 29523192 Free PMC article. Review.
-
Marine sponge microbial association: Towards disclosing unique symbiotic interactions.Mar Environ Res. 2018 Sep;140:169-179. doi: 10.1016/j.marenvres.2018.04.017. Epub 2018 Apr 27. Mar Environ Res. 2018. PMID: 29935729 Review.
Cited by
-
Trophic ecology of Angolan cold-water coral reefs (SE Atlantic) based on stable isotope analyses.Sci Rep. 2023 Jun 19;13(1):9933. doi: 10.1038/s41598-023-37035-x. Sci Rep. 2023. PMID: 37336945 Free PMC article.
-
Metagenomics-resolved genomics provides novel insights into chitin turnover, metabolic specialization, and niche partitioning in the octocoral microbiome.Microbiome. 2022 Sep 22;10(1):151. doi: 10.1186/s40168-022-01343-7. Microbiome. 2022. PMID: 36138466 Free PMC article.
-
Biodiversity of endosymbiont fungi associated with a marine sponge Lamellodysidea herbacea and their potential as antioxidant producers.3 Biotech. 2024 May;14(5):146. doi: 10.1007/s13205-024-03972-1. Epub 2024 May 3. 3 Biotech. 2024. PMID: 38706926 Free PMC article.
References
-
- Breitburg D, Levin LA, Oschlies A, Grégoire M, Chavez FP, Conley DJ, Garçon V, Gilbert D, Gutiérrez D, Isensee K, Jacinto GS, Limburg KE, Montes I, Naqvi SWA, Pitcher GC, Rabalais NN, Roman MR, Rose KA, Seibel BA, Telszewski M, Yasuhara M, Zhang J. 2018. Declining oxygen in the global ocean and coastal waters. Science 359:eaam7240. doi:10.1126/science.aam7240. - DOI - PubMed
-
- O’Boyle S 2020. Oxygen depletion in coastal waters and the open ocean, p 1–27. In Arias AH, Botte SE (ed), Coastal and deep ocean pollution, 1st ed ERC Press, Boca Raton, FL.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
