Conserved arginine residues in synaptotagmin 1 regulate fusion pore expansion through membrane contact
- PMID: 33536412
- PMCID: PMC7859215
- DOI: 10.1038/s41467-021-21090-x
Conserved arginine residues in synaptotagmin 1 regulate fusion pore expansion through membrane contact
Abstract
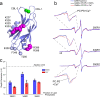
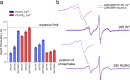
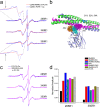
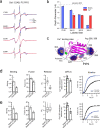
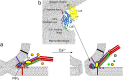
Synaptotagmin 1 is a vesicle-anchored membrane protein that functions as the Ca2+ sensor for synchronous neurotransmitter release. In this work, an arginine containing region in the second C2 domain of synaptotagmin 1 (C2B) is shown to control the expansion of the fusion pore and thereby the concentration of neurotransmitter released. This arginine apex, which is opposite the Ca2+ binding sites, interacts with membranes or membrane reconstituted SNAREs; however, only the membrane interactions occur under the conditions in which fusion takes place. Other regions of C2B influence the fusion probability and kinetics but do not control the expansion of the fusion pore. These data indicate that the C2B domain has at least two distinct molecular roles in the fusion event, and the data are consistent with a model where the arginine apex of C2B positions the domain at the curved membrane surface of the expanding fusion pore.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
A lever hypothesis for Synaptotagmin-1 action in neurotransmitter release.Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2417941121. doi: 10.1073/pnas.2417941121. Epub 2024 Dec 30. Proc Natl Acad Sci U S A. 2025. PMID: 39793049 Free PMC article.
-
Synaptotagmin-1 C2B domain interacts simultaneously with SNAREs and membranes to promote membrane fusion.Elife. 2016 Apr 15;5:e14211. doi: 10.7554/eLife.14211. Elife. 2016. PMID: 27083046 Free PMC article.
-
Evaluation of synaptotagmin-1 action models by all-atom molecular dynamics simulations.FEBS Open Bio. 2025 May;15(5):699-713. doi: 10.1002/2211-5463.13966. Epub 2025 Jan 15. FEBS Open Bio. 2025. PMID: 39815397 Free PMC article.
-
Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release.Trends Cell Biol. 2006 Jul;16(7):339-50. doi: 10.1016/j.tcb.2006.04.006. Epub 2006 May 12. Trends Cell Biol. 2006. PMID: 16698267 Review.
-
How does synaptotagmin trigger neurotransmitter release?Annu Rev Biochem. 2008;77:615-41. doi: 10.1146/annurev.biochem.77.062005.101135. Annu Rev Biochem. 2008. PMID: 18275379 Review.
Cited by
-
Synaptotagmin-1 C2B domains cooperatively stabilize the fusion stalk via a master-servant mechanism.Chem Sci. 2022 Feb 23;13(12):3437-3446. doi: 10.1039/d1sc06711g. eCollection 2022 Mar 24. Chem Sci. 2022. PMID: 35432859 Free PMC article.
-
Fluorescence Anisotropy for Monitoring cis- and trans-Membrane Interactions of Synaptotagmin-1.Methods Mol Biol. 2025;2887:175-182. doi: 10.1007/978-1-0716-4314-3_12. Methods Mol Biol. 2025. PMID: 39806154
-
Dynamic Formation of the Protein-Lipid Pre-fusion Complex.bioRxiv [Preprint]. 2024 May 11:2024.04.17.589983. doi: 10.1101/2024.04.17.589983. bioRxiv. 2024. Update in: Biophys J. 2024 Oct 15;123(20):3569-3586. doi: 10.1016/j.bpj.2024.09.009. PMID: 38659925 Free PMC article. Updated. Preprint.
-
Membrane lipids couple synaptotagmin to SNARE-mediated granule fusion in insulin-secreting cells.Mol Biol Cell. 2024 Mar 1;35(3):ar12. doi: 10.1091/mbc.E23-06-0225. Epub 2023 Dec 20. Mol Biol Cell. 2024. PMID: 38117594 Free PMC article.
-
Beyond the MUN domain, Munc13 controls priming and depriming of synaptic vesicles.Cell Rep. 2024 May 28;43(5):114026. doi: 10.1016/j.celrep.2024.114026. Epub 2024 May 21. Cell Rep. 2024. PMID: 38809756 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

