Biochemical and cellular characterisation of the Plasmodium falciparum M1 alanyl aminopeptidase (PfM1AAP) and M17 leucyl aminopeptidase (PfM17LAP)
- PMID: 33536500
- PMCID: PMC7858622
- DOI: 10.1038/s41598-021-82499-4
Biochemical and cellular characterisation of the Plasmodium falciparum M1 alanyl aminopeptidase (PfM1AAP) and M17 leucyl aminopeptidase (PfM17LAP)
Abstract
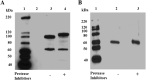
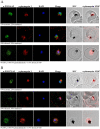
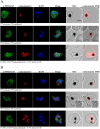
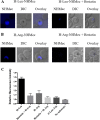
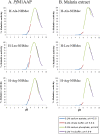
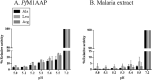
The Plasmodium falciparum M1 alanyl aminopeptidase and M17 leucyl aminopeptidase, PfM1AAP and PfM17LAP, are potential targets for novel anti-malarial drug development. Inhibitors of these aminopeptidases have been shown to kill malaria parasites in culture and reduce parasite growth in murine models. The two enzymes may function in the terminal stages of haemoglobin digestion, providing free amino acids for protein synthesis by the rapidly growing intra-erythrocytic parasites. Here we have performed a comparative cellular and biochemical characterisation of the two enzymes. Cell fractionation and immunolocalisation studies reveal that both enzymes are associated with the soluble cytosolic fraction of the parasite, with no evidence that they are present within other compartments, such as the digestive vacuole (DV). Enzyme kinetic studies show that the optimal pH of both enzymes is in the neutral range (pH 7.0-8.0), although PfM1AAP also possesses some activity (< 20%) at the lower pH range of 5.0-5.5. The data supports the proposal that PfM1AAP and PfM17LAP function in the cytoplasm of the parasite, likely in the degradation of haemoglobin-derived peptides generated in the DV and transported to the cytosol.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- World malaria report 2019. Licence: CC BY-NC-SA 3.0 IGO (World Health Organization, Geneva, 2019).
-
- Khera A, Mukherjee R. Artemisinin resistance: Cause for worry? J. Mar. Med. Soc. 2019;21:4. doi: 10.4103/jmms.jmms_43_18. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

