Analysis of amino acids, hydroxy acids, and amines in CR chondrites
- PMID: 33536738
- PMCID: PMC7839561
- DOI: 10.1111/maps.13586
Analysis of amino acids, hydroxy acids, and amines in CR chondrites
Abstract
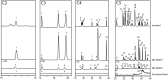
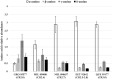
The abundances, relative distributions, and enantiomeric and isotopic compositions of amines, amino acids, and hydroxy acids in Miller Range (MIL) 090001 and MIL 090657 meteorites were determined. Chiral distributions and isotopic compositions confirmed that most of the compounds detected were indigenous to the meteorites and not the result of terrestrial contamination. Combined with data in the literature, suites of these compounds have now been analyzed in a set of six CR chondrites, spanning aqueous alteration types 2.0-2.8. Amino acid abundances ranged from 17 to 3300 nmol g-1 across the six CRs; hydroxy acid abundances ranged from 180 to 1800 nmol g-1; and amine abundances ranged from 40 to 2100 nmol g-1. For amino acids and amines, the weakly altered chondrites contained the highest abundances, whereas hydroxy acids were most abundant in the more altered CR2.0 chondrite. Because water contents in the meteorites are orders of magnitude greater than soluble organics, synthesis of hydroxy acids, which requires water, may be less affected by aqueous alteration than amines and amino acids that require nitrogen-bearing precursors. Two chiral amino acids that were plausibly extraterrestrial in origin were present with slight enantiomeric excesses: L-isovaline (~10% excess) and D-β-amino-n-butyric acid (~9% excess); further studies are needed to verify that the chiral excess in the latter compound is truly extraterrestrial in origin. The isotopic compositions of compounds reported here did not reveal definitive links between the different compound classes such as common synthetic precursors, but will provide a framework for further future in-depth analyses.
© 2020 The Authors. Meteoritics & Planetary Science published by Wiley Periodicals LLC on behalf of The Meteoritical Society (MET).
Figures
References
-
- Abreu N. M. 2016. Why is it so difficult to classify Renazzo‐type (CR) carbonaceous chondrites?—Implications from TEM observations of matrices for the sequences of aqueous alteration. Geochimica et Cosmochimica Acta 194:91–122.
-
- Alexander C. M. O'D., and Bowden R. 2018. Lewis Cliff (LEW) 85332 and Miller Range (MIL) 090001, a new grouplet that is distinct from the CR chondrites? (abstract #6063). 81st Annual Meeting of The Meteoritical Society.
-
- Alexander C. M. O'D., Howard K. T., Bowden R., and Fogel M. L. 2013. The classification of CM and CR chondrites using bulk H, C and N abundances and isotopic compositions. Geochimica et Cosmochimica Acta 123:244–260.
-
- Al‐waiz M., Mitchell S. C., Idle J. R., and Smith R. L. 1987. The metabolism of 14C‐labelled trimethylamine and its N‐oxide in man. Xenobiotica 17:551–558. - PubMed
-
- Aponte J. C., Dworkin J. P., and Elsila J. E. 2014. Assessing the origins of aliphatic amines in the Murchison meteorite from their compound‐specific carbon isotopic ratios and enantiomeric composition. Geochimica et Cosmochimica Acta 141:331–345.
LinkOut - more resources
Full Text Sources
