Tezepelumab as an Emerging Therapeutic Option for the Treatment of Severe Asthma: Evidence to Date
- PMID: 33536746
- PMCID: PMC7850420
- DOI: 10.2147/DDDT.S250825
Tezepelumab as an Emerging Therapeutic Option for the Treatment of Severe Asthma: Evidence to Date
Abstract
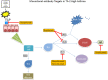
Asthma is a complex heterogeneous disease defined by chronic inflammation of the airways. Patients present with wheezing, chest tightness, cough and shortness of breath. Bronchial hyperresponsiveness and variable expiratory airflow limitation are hallmark features. About 3.6-6.1% of patients, despite receiving high-dose inhaled corticosteroids (ICS) and a second controller medication, report persistent symptoms referred to as severe asthma. Uncontrolled severe asthma is associated with increased mortality, morbidity, diminished quality of life and increased health expenditures. The development of modern biological therapy has revolutionized severe asthma treatment. By targeting specific chemokines, asthma control has drastically improved, resulting in better quality of life, less emergency department visits and inpatient admissions, and decreased chronic systemic corticosteroid utilization. Despite these advances, there remains a subset of asthma patients who remain symptomatic with poor quality of life and heavy utilization of the healthcare system. Recently attention has been given to pharmaceutical therapy directed at receptors and cytokines on the epithelial layer of the lung referred to as "alarmins". Thymic stromal lymphopoietin (TSLP) is an interleukin-7-like receptor family found on the epithelial layer of the lung that releases a cytokine cascade inducing eosinophilic inflammation, mucus production and airflow obstruction in asthmatics. Tezepelumab is the first investigational monoclonal antibody that inhibits TSLP. Proof of concept study and phase IIb studies demonstrated reduced asthma exacerbations, improvement in quality of life, less decline in FEV1 and decrease in biochemical inflammatory markers in comparison to placebo. It is presently undergoing three phase III studies and an additional phase II study.
Keywords: biological therapy; eosinophilic asthma; monoclonal antibody; non-eosinophilic asthma; severe uncontrolled asthma; tezepelumab.
© 2021 Dorey-Stein and Shenoy.
Conflict of interest statement
Dr. Shenoy has performed advisory work on behalf of AstraZeneca. The authors report no other conflicts of interest in this work.
Figures
References
-
- Anaptysbio reports top-line data from interim analysis of eclipse phase 2 clinical trial of etokimab in chronic rhinosinusitis with nasal polyps; 2020. Available from: https://www.globenewswire.com/Website. Accessed September13, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical