A Retrospective Observational Study of Anlotinib in Patients with Platinum-Resistant or Platinum-Refractory Epithelial Ovarian Cancer
- PMID: 33536747
- PMCID: PMC7850384
- DOI: 10.2147/DDDT.S286529
A Retrospective Observational Study of Anlotinib in Patients with Platinum-Resistant or Platinum-Refractory Epithelial Ovarian Cancer
Abstract
Objective: Anlotinib, an oral small-molecular tyrosine kinase inhibitor (TKI) on tumor angiogenesis and growth, has a wide spectrum of inhibitory effects on targets such as vascular endothelial growth factor receptors 2/3 (VEGFR2/3), etc. The efficacy and safety of anlotinib in the treatment of platinum-resistant or platinum-refractory ovarian cancer were evaluated.
Patients and methods: Patients with platinum-resistant or platinum-refractory ovarian cancer that treated with anlotinib in the Affiliated Cancer Hospital of Zhengzhou University from May 2018 to March 2020 were included. Medical records were reviewed in terms of objective response, survival outcomes, and safety.
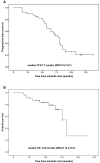
Results: A total of 38 patients were analyzed. The median progression-free survival and the median overall survival were 7.7 months (95% CI: 6.7-8.7) and 16.5 months (95% CI: 13.3-19.7), respectively. About 17 patients received anlotinib monotherapy, and the median progression-free survival was 7.7 months (95% CI: 6.3-9.1). A total of 19 cases received anlotinib plus chemotherapy with a median progression-free survival of 8.0 months (95% CI: 4.8-11.2). A total of 2 cases received anlotinib plus anti-PD-1 antibody pembrolizumab, and 1 case had partial response, the other progressive disease. The objective response rate was 42.1% while the disease control rate was 86.8%. A total of 5 patients experienced dose reduction from 12 mg to 10 mg because of adverse effects. The most common adverse effects were hypertension (31.6%), fatigue (28.9%), anorexia (26.3%) and hand-foot syndrome (23.7%). No treatment-related deaths were recorded.
Conclusion: Anlotinib produced moderate improvements in progression-free survival and overall survival in patients with platinum-resistant or platinum-refractory ovarian cancer. It indicates that anlotinib maybe a new treatment option for patients with platinum-resistant or platinum-refractory ovarian cancer.
Keywords: angiogenesis; anlotinib; ovarian cancer; platinum-resistant.
© 2021 Cui et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018[J]. CA Cancer J Clin. 2018;68(1):7–30. - PubMed
-
- Ozols RF. Systemic therapy for ovarian cancer: current status and new treatments[J]. Semin Oncol. 2006;33(2 Suppl 6):S3–S11. - PubMed
-
- Pujade-Lauraine E, Combe P. Recurrent ovarian cancer[J]. Ann Oncol. 2016;27(Suppl 1):i63–i65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical