Animal Models of Metabolic Disorders in the Study of Neurodegenerative Diseases: An Overview
- PMID: 33536868
- PMCID: PMC7848140
- DOI: 10.3389/fnins.2020.604150
Animal Models of Metabolic Disorders in the Study of Neurodegenerative Diseases: An Overview
Abstract
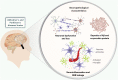
The incidence of metabolic disorders, as well as of neurodegenerative diseases-mainly the sporadic forms of Alzheimer's and Parkinson's disease-are increasing worldwide. Notably, obesity, diabetes, and hypercholesterolemia have been indicated as early risk factors for sporadic forms of Alzheimer's and Parkinson's disease. These conditions share a range of molecular and cellular features, including protein aggregation, oxidative stress, neuroinflammation, and blood-brain barrier dysfunction, all of which contribute to neuronal death and cognitive impairment. Rodent models of obesity, diabetes, and hypercholesterolemia exhibit all the hallmarks of these degenerative diseases, and represent an interesting approach to the study of the phenotypic features and pathogenic mechanisms of neurodegenerative disorders. We review the main pathological aspects of Alzheimer's and Parkinson's disease as summarized in rodent models of obesity, diabetes, and hypercholesterolemia.
Keywords: Alzheimer’s disease; Parkinson’s disease; diabetes; hypercholesterolemia; neurodegeneration; obesity; rodent models.
Copyright © 2021 de Bem, Krolow, Farias, de Rezende, Gelain, Moreira, Duarte and de Oliveira.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Ahlqvist E., Storm P., Käräjämäki A., Martinell M., Dorkhan M., Carlsson A., et al. (2018). Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 6 361–369. 10.1016/S2213-8587(18)30051-2 - DOI - PubMed
-
- Arcego D. M., Krolow R., Lampert C., Toniazzo A. P., Berlitz C., Lazzaretti C., et al. (2016). Early life adversities or high fat diet intake reduce cognitive function and alter BDNF signaling in adult rats: interplay of these factors changes these effects. Int. J. Dev. Neurosci. 50 16–25. 10.1016/j.ijdevneu.2016.03.001 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

