Transcriptomic Coupling of PKP2 With Inflammatory and Immune Pathways Endogenous to Adult Cardiac Myocytes
- PMID: 33536940
- PMCID: PMC7849609
- DOI: 10.3389/fphys.2020.623190
Transcriptomic Coupling of PKP2 With Inflammatory and Immune Pathways Endogenous to Adult Cardiac Myocytes
Abstract
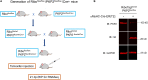
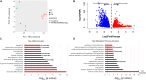
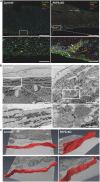
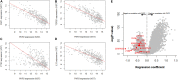
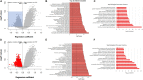
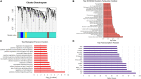
Plakophilin-2 (PKP2) is classically defined as a component of the desmosome. Besides its role in cell-cell adhesion, PKP2 can modulate transcription through intracellular signals initiated at the site of cell-cell contact. Mutations in PKP2 associate with arrhythmogenic right ventricular cardiomyopathy (ARVC). Recent data demonstrate that inflammation plays a key role in disease progression; other results show an abundance of anti-heart antibodies in patients with confirmed diagnosis of ARVC. Here, we test the hypothesis that, in adult cardiac myocytes, PKP2 transcript abundance is endogenously linked to the abundance of transcripts participating in the inflammatory/immune response. Cardiac-specific, tamoxifen (TAM)-activated PKP2-knockout mice (PKP2cKO) were crossed with a RiboTag line to allow characterization of the ribosome-resident transcriptome of cardiomyocytes after PKP2 knockdown. Data were combined with informatics analysis of human cardiac transcriptome using GTEx. Separately, the presence of non-myocyte cells at the time of analysis was assessed by imaging methods. We identified a large number of transcripts upregulated consequent to PKP2 deficiency in myocytes, inversely correlated with PKP2 abundance in human transcriptomes, and part of functional pathways associated with inflammatory/immune responses. Our data support the concept that PKP2 is transcriptionally linked, in cardiac myocytes, to genes coding for host-response molecules even in the absence of exogenous triggers. Targeted anti-inflammatory therapy may be effective in ARVC.
Keywords: GTEx; arrhythmogenic cardiomyopathy; inflammation/immune response; plakophilin-2; transcriptome.
Copyright © 2021 Pérez-Hernández, Marrón-Liñares, Schlamp, Heguy, van Opbergen, Mezzano, Zhang, Liang, Cerrone and Delmar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Basso C., Corrado D., Marcus F. I., Nava A., Thiene G. (2009). Arrhythmogenic right ventricular cardiomyopathy. Lancet 373 1289–1300. - PubMed
-
- Bockstahler M., Fischer A., Goetzke C. C., Neumaier H. L., Sauter M., Kespohl M., et al. (2020). Heart-Specific Immune Responses in an Animal Model of AutoimmuneRelated Myocarditis Mitigated by an Immunoproteasome Inhibitor and Genetic Ablation. Circulation 141 1885–1902. 10.1161/circulationaha.119.043171 - DOI - PubMed
-
- Caforio A. L. P., Re F., Avella A., Marcolongo R., Baratta P., Seguso M., et al. (2020). Evidence From Family Studies for Autoimmunity in Arrhythmogenic Right Ventricular Cardiomyopathy: Associations of Circulating Anti-Heart and Anti-Intercalated Disk Autoantibodies With Disease Severity and Family History. Circulation 141 1238–1248. 10.1161/circulationaha.119.043931 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

