Epigenetic Regulation Associated With Sirtuin 1 in Complications of Diabetes Mellitus
- PMID: 33537003
- PMCID: PMC7848207
- DOI: 10.3389/fendo.2020.598012
Epigenetic Regulation Associated With Sirtuin 1 in Complications of Diabetes Mellitus
Abstract
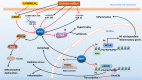
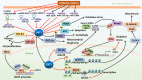
Diabetes mellitus (DM) has been one of the largest health concerns of the 21st century due to the serious complications associated with the disease. Therefore, it is essential to investigate the pathogenesis of DM and develop novel strategies to reduce the burden of diabetic complications. Sirtuin 1 (SIRT1), a nicotinamide adenosine dinucleotide (NAD+)-dependent deacetylase, has been reported to not only deacetylate histones to modulate chromatin function but also deacetylate numerous transcription factors to regulate the expression of target genes, both positively and negatively. SIRT1 also plays a crucial role in regulating histone and DNA methylation through the recruitment of other nuclear enzymes to the chromatin. Furthermore, SIRT1 has been verified as a direct target of many microRNAs (miRNAs). Recently, numerous studies have explored the key roles of SIRT1 and other related epigenetic mechanisms in diabetic complications. Thus, this review aims to present a summary of the rapidly growing field of epigenetic regulatory mechanisms, as well as the epigenetic influence of SIRT1 on the development and progression of diabetic complications, including cardiomyopathy, nephropathy, and retinopathy.
Keywords: SIRT1; deacetylation; diabetes mellitus; diabetic complications; epigenetics.
Copyright © 2021 Wang(a), Wang, Wang(b), Xiao, Guo, Tang, Zhang and Gu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor declared a shared affiliation with several of the authors JW(a), JW(b), MX, YG, YT, and JG at time of review.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

