PfAP2-G2 Is Associated to Production and Maturation of Gametocytes in Plasmodium falciparum via Regulating the Expression of PfMDV-1
- PMID: 33537025
- PMCID: PMC7848025
- DOI: 10.3389/fmicb.2020.631444
PfAP2-G2 Is Associated to Production and Maturation of Gametocytes in Plasmodium falciparum via Regulating the Expression of PfMDV-1
Abstract
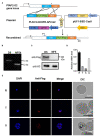
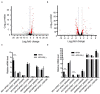
Gametocyte is the sole form of the Plasmodium falciparum which is transmissible to the mosquito vector. Here, we report that an Apicomplexan Apetala2 (ApiAP2) family transcription factor, PfAP2-G2 (Pf3D7_1408200), plays a role in the development of gametocytes in P. falciparum by regulating the expression of PfMDV-1 (Pf3D7_1216500). Reverse transcriptase-quantitative PCR (RT-qPCR) analysis showed that PfAP2-G2 was highly expressed in the ring stage. Indirect immunofluorescence assay showed nuclear localization of PfAP2-G2 in asexual stages. The knockout of PfAP2-G2 led to a ~95% decrease in the number of mature gametocytes with a more substantial influence on the production and maturation of the male gametocytes, resulting in a higher female/male gametocyte ratio. To test the mechanism of this phenotype, RNA-seq and RT-qPCR showed that disruption of PfAP2-G2 led to the down-regulation of male development gene-1 (PfMDV-1) in asexual stages. We further found that PfAP2-G2 was enriched at the transcriptional start site (TSS) of PfMDV-1 by chromatin immunoprecipitation and qPCR assay in both ring stage and schizont stage, which demonstrated that PfMDV-1 is one of the targets of PfAP2-G2. In addition, RT-qPCR also showed that PfAP2-G (Pf3D7_1222600), the master regulator for sexual commitment, was also down-regulated in the PfAP2-G2 knockout parasites in the schizont stage, but no change in the ring stage. This phenomenon suggested that PfAP2-G2 played a role at the asexual stage for the development of parasite gametocytes and warrants further investigations in regulatory pathways of PfAP2-G2.
Keywords: Plasmodium falciparum; gametocytes; malaria; sexual development; transcriptional factor.
Copyright © 2021 Xu, Qiao, Wen, Bi, Chen, Huang, Cui, Guo and Cao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

