Molecular Mechanisms of the 1-Aminocyclopropane-1-Carboxylic Acid (ACC) Deaminase Producing Trichoderma asperellum MAP1 in Enhancing Wheat Tolerance to Waterlogging Stress
- PMID: 33537050
- PMCID: PMC7847992
- DOI: 10.3389/fpls.2020.614971
Molecular Mechanisms of the 1-Aminocyclopropane-1-Carboxylic Acid (ACC) Deaminase Producing Trichoderma asperellum MAP1 in Enhancing Wheat Tolerance to Waterlogging Stress
Abstract
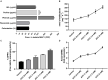
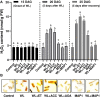
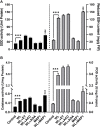
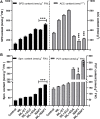
Waterlogging stress (WS) induces ethylene (ET) and polyamine (spermine, putrescine, and spermidine) production in plants, but their reprogramming is a decisive element for determining the fate of the plant upon waterlogging-induced stress. WS can be challenged by exploring symbiotic microbes that improve the plant's ability to grow better and resist WS. The present study deals with identification and application of 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase-producing fungal endophyte Trichoderma asperellum (strain MAP1), isolated from the roots of Canna indica L., on wheat growth under WS. MAP1 positively affected wheat growth by secreting phytohormones/secondary metabolites, strengthening the plant's antioxidant system and influencing the physiology through polyamine production and modulating gene expression. MAP1 inoculation promoted yield in comparison to non-endophyte inoculated waterlogged seedlings. Exogenously applied ethephon (ET synthesis inducer) and 1-aminocyclopropane carboxylic acid (ACC; ET precursor) showed a reduction in growth, compared to MAP1-inoculated waterlogged seedlings, while amino-oxyacetic acid (AOA; ET inhibitor) application reversed the negative effect imposed by ET and ACC, upon waterlogging treatment. A significant reduction in plant growth rate, chlorophyll content, and stomatal conductance was noticed, while H2O2, MDA production, and electrolyte leakage were increased in non-inoculated waterlogged seedlings. Moreover, in comparison to non-inoculated waterlogged wheat seedlings, MAP1-inoculated waterlogged wheat exhibited antioxidant-enzyme activities. In agreement with the physiological results, genes associated with the free polyamine (PA) biosynthesis were highly induced and PA content was abundant in MAP1-inoculated seedlings. Furthermore, ET biosynthesis/signaling gene expression was reduced upon MAP1 inoculation under WS. Briefly, MAP1 mitigated the adverse effect of WS in wheat, by reprogramming the PAs and ET biosynthesis, which leads to optimal stomatal conductance, increased photosynthesis, and membrane stability as well as reduced ET-induced leaf senescence.
Keywords: ACC deaminase enzyme; Trichoderma asperellum; biofertilizer; endophytic fungus; ethylene; polyamines; waterlogging stress; wheat.
Copyright © 2021 Rauf, Awais, Ud-Din, Ali, Gul, Rahman, Hamayun and Arif.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Aspergillus nomiae and fumigatus Ameliorating the Hypoxic Stress Induced by Waterlogging through Ethylene Metabolism in Zea mays L.Microorganisms. 2023 Aug 7;11(8):2025. doi: 10.3390/microorganisms11082025. Microorganisms. 2023. PMID: 37630585 Free PMC article.
-
1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase-containing rhizobacteria protect Ocimum sanctum plants during waterlogging stress via reduced ethylene generation.Plant Physiol Biochem. 2012 Sep;58:227-35. doi: 10.1016/j.plaphy.2012.07.008. Epub 2012 Jul 20. Plant Physiol Biochem. 2012. PMID: 22846334
-
Mechanisms of the IAA and ACC-deaminase producing strain of Trichoderma longibrachiatum T6 in enhancing wheat seedling tolerance to NaCl stress.BMC Plant Biol. 2019 Jan 11;19(1):22. doi: 10.1186/s12870-018-1618-5. BMC Plant Biol. 2019. PMID: 30634903 Free PMC article.
-
Plant Growth Promotion Under Water: Decrease of Waterlogging-Induced ACC and Ethylene Levels by ACC Deaminase-Producing Bacteria.Front Microbiol. 2018 May 25;9:1096. doi: 10.3389/fmicb.2018.01096. eCollection 2018. Front Microbiol. 2018. PMID: 29887854 Free PMC article. Review.
-
ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops.Microbiol Res. 2020 May;235:126439. doi: 10.1016/j.micres.2020.126439. Epub 2020 Feb 15. Microbiol Res. 2020. PMID: 32097862 Review.
Cited by
-
ACC deaminase-producing endophytic fungal consortia promotes drought stress tolerance in M.oleifera by mitigating ethylene and H2O2.Front Plant Sci. 2022 Dec 22;13:967672. doi: 10.3389/fpls.2022.967672. eCollection 2022. Front Plant Sci. 2022. PMID: 36618664 Free PMC article.
-
Growth-Promoting Characteristics of Fungal and Bacterial Endophytes Isolated from a Drought-Tolerant Mint Species Endostemon obtusifolius (E. Mey. ex Benth.) N. E. Br.Plants (Basel). 2023 Feb 1;12(3):638. doi: 10.3390/plants12030638. Plants (Basel). 2023. PMID: 36771720 Free PMC article.
-
Aspergillus nomiae and fumigatus Ameliorating the Hypoxic Stress Induced by Waterlogging through Ethylene Metabolism in Zea mays L.Microorganisms. 2023 Aug 7;11(8):2025. doi: 10.3390/microorganisms11082025. Microorganisms. 2023. PMID: 37630585 Free PMC article.
-
Looking for Resistance to Soft Rot Disease of Potatoes Facing Environmental Hypoxia.Int J Mol Sci. 2024 Mar 28;25(7):3757. doi: 10.3390/ijms25073757. Int J Mol Sci. 2024. PMID: 38612570 Free PMC article. Review.
-
Exogenous treatment with melatonin enhances waterlogging tolerance of kiwifruit plants.Front Plant Sci. 2022 Dec 9;13:1081787. doi: 10.3389/fpls.2022.1081787. eCollection 2022. Front Plant Sci. 2022. PMID: 36570925 Free PMC article.
References
-
- Asada K. (1992). Ascorbate peroxidase–a hydrogen peroxide−scavenging enzyme in plants. Physiol. Plant. 85 235–241. 10.1111/j.1399-3054.1992.tb04728.x - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

