Overall survival of pancreatic ductal adenocarcinoma is doubled by Aldh7a1 deletion in the KPC mouse
- PMID: 33537098
- PMCID: PMC7847681
- DOI: 10.7150/thno.53935
Overall survival of pancreatic ductal adenocarcinoma is doubled by Aldh7a1 deletion in the KPC mouse
Abstract
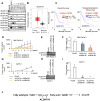
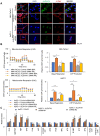
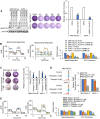
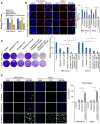
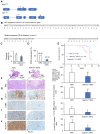
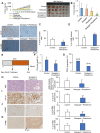
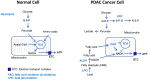
Rationale: The activity of aldehyde dehydrogenase 7A1 (ALDH7A1), an enzyme that catalyzes the lipid peroxidation of fatty aldehydes was found to be upregulated in pancreatic ductal adenocarcinoma (PDAC). ALDH7A1 knockdown significantly reduced tumor formation in PDAC. We raised a question how ALDH7A1 contributes to cancer progression. Methods: To answer the question, the role of ALDH7A1 in energy metabolism was investigated by knocking down and knockdown gene in mouse model, because the role of ALDH7A1 has been reported as a catabolic enzyme catalyzing fatty aldehyde from lipid peroxidation to fatty acid. Oxygen consumption rate (OCR), ATP production, mitochondrial membrane potential, proliferation assay and immunoblotting were performed. In in vivo study, two human PDAC cell lines were used for pre-clinical xenograft model as well as spontaneous PDAC model of KPC mice was also employed for anti-cancer therapeutic effect. Results:ALDH7A1 knockdown significantly reduced tumor formation with reduction of OCR and ATP production, which was inversely correlated with increase of 4-hydroxynonenal. This implies that ALDH7A1 is critical to process fatty aldehydes from lipid peroxidation. Overall survival of PDAC is doubled by cross breeding of KPC (KrasG12D; Trp53R172H; Pdx1-Cre) and Aldh7a1-/- mice. Conclusion: Inhibitions of ALDH7A1 and oxidative phosphorylation using gossypol and phenformin resulted in a regression of tumor formation in xenograft mice model and KPC mice model.
Keywords: ALDH7A1; KPC mice model; cancer metabolism; oxidative phosphorylation complex I; pancreatic ductal adenocarcinoma.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Le Large TY, Mato Prado M, Krell J, Bijlsma MF, Meijer LL, Kazemier G. et al. Bioinformatic analysis reveals pancreatic cancer molecular subtypes specific to the tumor and the microenvironment. Expert Rev Mol Diagn. 2016;16:733–6. - PubMed
-
- Maurel J, Sanchez-Cabus S, Laquente B, Gaba L, Visa L, Fabregat J. et al. Outcomes after neoadjuvant treatment with gemcitabine and erlotinib followed by gemcitabine-erlotinib and radiotherapy for resectable pancreatic cancer (GEMCAD 10-03 trial) Cancer Chemother Pharmacol. 2018;82:935–43. - PubMed
-
- Saif MW. Advancements in the management of pancreatic cancer: 2013. JOP. 2013;14:112–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

