Ovarian hormones-autophagy-immunity axis in menstruation and endometriosis
- PMID: 33537101
- PMCID: PMC7847674
- DOI: 10.7150/thno.55241
Ovarian hormones-autophagy-immunity axis in menstruation and endometriosis
Abstract
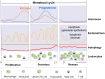
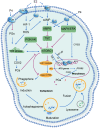
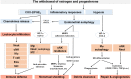
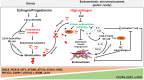
Menstruation occurs in few species and involves a cyclic process of proliferation, breakdown and regeneration under the control of ovarian hormones. Knowledge of normal endometrial physiology, as it pertains to the regulation of menstruation, is essential to understand disorders of menstruation. Accumulating evidence indicates that autophagy in the endometrium, under the regulation of ovarian hormones, can result in the infiltration of immune cells, which plays an indispensable role in the endometrium shedding, tissue repair and prevention of infections during menstruation. In addition, abnormal autophagy levels, together with resulting dysregulated immune system function, are associated with the pathogenesis and progression of endometriosis. Considering its potential value of autophagy as a target for the treatment of menstrual-related and endometrium-related disorders, we review the activity and function of autophagy during menstrual cycles. The role of the estrogen/progesterone-autophagy-immunity axis in endometriosis are also discussed.
Keywords: NK cell; autophagy; endometrium; estrogen; macrophage; menstruation; neutrophil; progesterone.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Jarrell J. The significance and evolution of menstruation. Best Pract Res Clin Obstet Gynaecol. 2018;50:18–26. - PubMed
-
- Azlan A, Salamonsen LA, Hutchison J, Evans J. Endometrial inflammasome activation accompanies menstruation and may have implications for systemic inflammatory events of the menstrual cycle. Hum Reprod. 2020;35:1363–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

