Retinoic and ascorbic acids induce osteoblast differentiation from human dental pulp mesenchymal stem cells
- PMID: 33537186
- PMCID: PMC7840990
- DOI: 10.1016/j.jobcr.2021.01.002
Retinoic and ascorbic acids induce osteoblast differentiation from human dental pulp mesenchymal stem cells
Abstract
Previous studies have suggested an important role of retinoic acid (RA) and ascorbic acid (AA) in the stimulation of osteoblastic differentiation; however, the function of RA and AA in the osteogenic differentiation from human dental pulp (hDPSCs) remains unclear.
Objective: This in vitro study investigated the effects of RA and AA on the differentiation of osteoblast from hDPSCs.
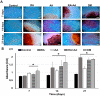
Methods: hDPSCs were treated with different doses of RA and AA, separately or in combination (RA + AA). Morphology and cell proliferation were assessed. Osteoblast differentiation was evaluated by alizarin red, alkaline phosphatase staining, and RUNX2 gene expression.
Results: A significant reduction was observed in the number of cells treated with RA (26%) and RA + AA (30%) after 12 days of treatment. AA treatment alone induced a 12% reduction in the number of cells. Morphologically, the cells treated with RA and RA + AA were larger and more elongated than the control cells. A mesh pattern was observed in cells treated with AA. Numerous calcified nodules were present in cells treated with RA, AA, and RA + AA. This coincided with increased expression of RUNX2 and high alkaline phosphatase staining levels.
Conclusions: hDPSCs treated with RA and RA + AA showed significant reduction in proliferation, detectable morphological changes, and expression of the key differentiation gene RUNX2, consistent with an osteoblast phenotype. AA induced morphological changes and early formation of calcified nodules. RA had a predominant effect when AA and RA were used together.
Keywords: Ascorbic acid; Cell differentiation; Mesenchymal stem cells; Osteoblasts; Retinoic acid.
© 2021 Craniofacial Research Foundation. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures




References
-
- Almpani K., Kantarci A. Nonsurgical methods for the acceleration of the orthodontic tooth movement. Front Oral Biol. 2016;18:80–91. - PubMed
-
- Motoji H., To M., Hidaka K., Matsuo M. Vitamin C and eggshell membrane facilitate orthodontic tooth movement and induce histological changes in the periodontal tissue. J Oral Biosci. 2020;62(1):80–87. - PubMed
-
- Weng Z., Wang C., Zhang C. All-trans retinoic acid promotes osteogenic differentiation and bone consolidation in a rat distraction osteogenesis model. Calcif Tissue Int. 2019;104(3):320–330. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

