miRNAs and Müller Glia Reprogramming During Retina Regeneration
- PMID: 33537319
- PMCID: PMC7848101
- DOI: 10.3389/fcell.2020.632632
miRNAs and Müller Glia Reprogramming During Retina Regeneration
Abstract
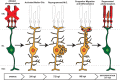
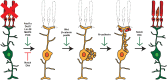
The use of model systems that are capable of robust, spontaneous retina regeneration has allowed for the identification of genetic pathways and components that are required for retina regeneration. Complemented by mouse models in which retina regeneration can be induced after forced expression of key factors, altered chromatin accessibility, or inhibition of kinase/signaling cascades, a clearer picture of the key regulatory events that control retina regeneration is emerging. In all cases, Müller glia (MG) serve as an adult retinal stem cell that must be reprogrammed to allow for regeneration, with the end goal being to understand why regenerative pathways are blocked in mammals, but spontaneous in other vertebrates such as zebrafish. miRNAs have emerged as key gene regulatory molecules that control both development and regeneration in vertebrates. Here, we focus on a small subset of miRNAs that control MG reprogramming during retina regeneration and have the potential to serve as therapeutic targets for treatment of visual disorders and damage.
Keywords: Müller glia; miRNA; regeneration; retina; zebrafish.
Copyright © 2021 Konar, Ferguson, Flickinger, Kent and Patton.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

