An Overview of Nucleic Acid Testing for the Novel Coronavirus SARS-CoV-2
- PMID: 33537322
- PMCID: PMC7848129
- DOI: 10.3389/fmed.2020.571709
An Overview of Nucleic Acid Testing for the Novel Coronavirus SARS-CoV-2
Abstract
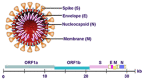
In this note we analyze the problems in the nucleic acid testing (NAT) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and we also give some suggestions for improving the accuracy of NAT diagnosis. NAT testing is considered to be the diagnostic "gold standard"; at present there are few reviews on NAT for SARS-CoV-2. Moreover, many false-negative results always appear in the procedure of detecting, which has affected early diagnosis of the disease and brought a great challenge to mitigation and containment of the pandemic. In conclusion, comprehensive analyses of serological and imaging findings should be performed to guide the formulation of an accurate clinical diagnosis, treatment plan, and monitoring therapeutic efficacy, in an effort to achieve early diagnosis, containment, and treatment of the disease, thereby effectively reducing progression of the pandemic. This article presents a literature overview of SARS-CoV-2 nucleic acid testing, aiming to provide support for clinicians.
Keywords: COVID-19; SARS-CoV-2; nucleic acid testing; qRT-PCR; specimen.
Copyright © 2021 Wang, Li, Zhao, Li and Ai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous