Rheumatic Heart Valve Disease Pathophysiology and Underlying Mechanisms
- PMID: 33537348
- PMCID: PMC7848031
- DOI: 10.3389/fcvm.2020.612716
Rheumatic Heart Valve Disease Pathophysiology and Underlying Mechanisms
Abstract
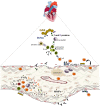
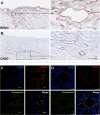
Rheumatic heart valve disease (RHVD) is a post-infectious sequel of acute rheumatic fever resulting from an abnormal immune response to a streptococcal pharyngitis that triggers valvular damage. RHVD is the leading cause of cardiovascular death in children and young adults, mainly in women from low and middle-income countries. It is known that long-term inflammation and high degree of fibrosis leads to valve dysfunction due to anatomic disruption of the valve apparatus. However, since public and private investments in RHVD studies are practically inexistent the number of publications is scarce. This disease shows different natural history and clinical presentations as compared to other degenerative heart valve diseases. Although more than five decades passed after the pioneering studies on the pathogenesis of RHVD, it is still unclear how self-tolerance mechanisms fail in this disease, and how humoral and cellular inflammatory responses are interconnected. Despite that pathological mechanisms have been already proposed for RHVD, none of them are able to explain the preferential involvement of the mitral valve. This review focuses on pathophysiology and underlying mechanisms of RHVD.
Keywords: autoimmunity; inflammation; mitral valve; pathogenesis; rheumatic heart disease.
Copyright © 2021 Passos, Nunes and Aikawa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

